Novel application of vinblastine and its analogue
A technology of vinblastine and substances, which is applied to the new application field of vinblastine substances, can solve problems such as studies that have not yet been reported, and achieve the effects of significant differences in efficacy and good efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
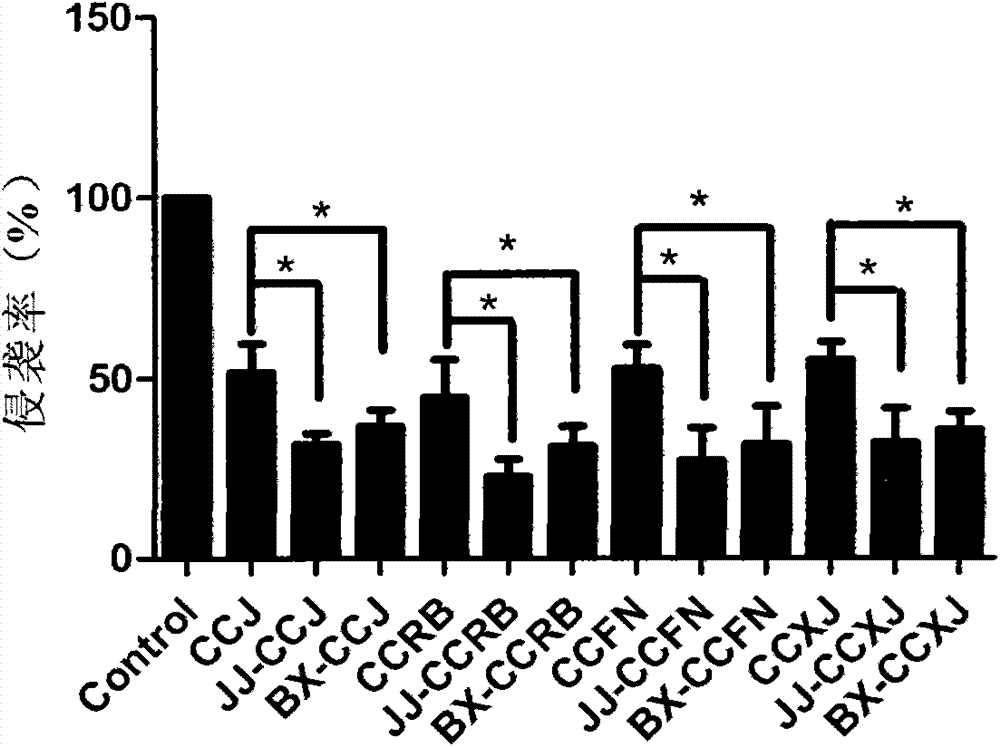
[0038] Example 1 Inhibitory effect of vinblastine derivatives on the invasion ability of human vascular endothelial cells HUVECs
[0039] Experimental method: HUVECs cells in the logarithmic growth phase were digested, centrifuged, counted, and 5×10 6 / mL were respectively inoculated in Transwell chambers covered with Matrigel Matrigel (BD Company), 0.1 mL per hole, placed in 5% CO 2 , 37 ℃ cell incubator after incubation for 24 hours, set up the blank control group and the drug treatment group, the drug group were added vinblastine compounds vinblastine (CCJ), vinorelbine (CCRB), vinflunine (CCFN) and vinblastine Neosine (CCXJ) and vinblastine derivatives hydrazine vinblastine (JJ-CCJ), vinblastine dipeptide (BX-CCJ), hydrazinolysis vinorelbine (JJ-CCRB), vinorelbine dipeptide (BX- CCRB), hydrazinolyzed vinflunine (JJ-CCFN), vinflunine dipeptide (BX-CCFN), hydrazinolyzed vincristine (JJ-CCXJ) and vincristine dipeptide (BX-CCXJ), final drug concentration Both were 100pmol / L....
Embodiment 2
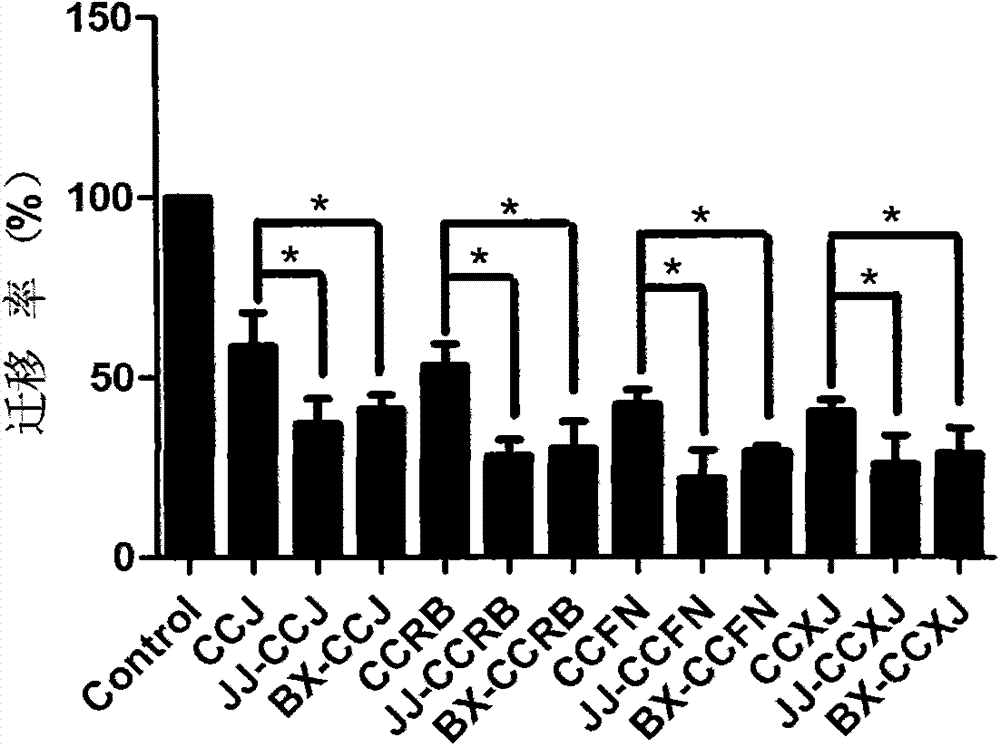
[0042] Example 2 Inhibitory Effect of Vinblastine Derivatives on the Migration Ability of Human Vascular Endothelial Cells HUVECs
[0043] Experimental method: Take HUVECs cells in logarithmic growth phase, wash and centrifuge with PBS, and resuspend the cells in serum-free medium. Will 2×10 6 Cells / mL were inoculated in Transwell chambers, 100 μL per well, and a blank control group and a drug treatment group were set up. The vinblastine compounds vinblastine (CCJ), vinorelbine (CCRB), vinflunine (CCFN ) and vincristine (CCXJ) and vinblastine derivatives hydrazinolyzed vinblastine (JJ-CCJ), vinblastine dipeptide (BX-CCJ), hydrazinolyzed vinorelbine (JJ-CCRB), vinorelbine dipeptide (BX-CCRB), hydrazinolyzed vinflunine (JJ-CCFN), vinflunine dipeptide (BX-CCFN), hydrazinolyzed vincristine (JJ-CCXJ) and vincristine dipeptide (BX-CCXJ), The final drug concentration was 100 pmol / L, and 700 μL of 1640 culture solution containing 10% newborn bovine serum was added to the lower chamb...
Embodiment 3
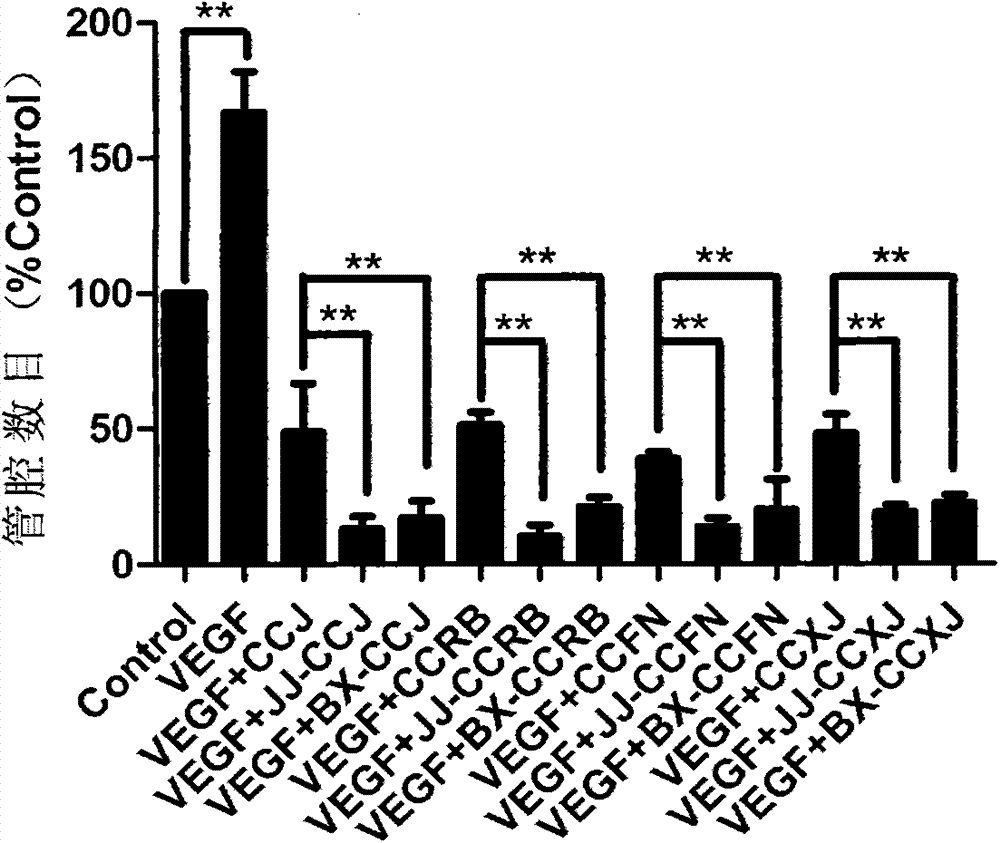
[0047] Example 3 Inhibitory Effect of Vinblastine Derivatives on Lumen Formation of Human Vascular Endothelial Cells HUVECs
[0048] Experimental method: Spread Matrigel in a pre-cooled 48-well plate, add 100 μL to each well, and place it in a 37°C cell culture incubator for 20 minutes. After the Matrigel is solidified, add 1×10 5 HUVECs cells / well, the cell culture medium was pre-added with vinblastine compounds and vinblastine derivatives JJ-CCJ, BX-CCJ, JJ-CCRB, BX-CCRB, JJ-CCFN, BX-CCFN, JJ-CCXJ and BX -CCXJ (final concentration is 100pmol / L) and vascular endothelial growth factor (VEGF) (final concentration is 100ng / mL), the wells without drug and VEGF are used as blank control group. After continuing to culture for 8 hours, observe the formation of the lumen under an inverted microscope and take pictures.
[0049] The result is as image 3 .
[0050] The experimental results showed that vinblastine compounds, hydrazinolyzed vinblastine compounds and vinblastine dipep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com