Application of Rhodococcus erythrococcus in degrading aflatoxin b1 in feed or its raw materials
A technology of Rhodococcus erythrococcus and aflatoxin, applied in the direction of bacteria, microorganism-based methods, microorganisms, etc., can solve the problems of complex degradation operation process, difficulty in large-scale implementation, and low efficiency of toxin degradation, and achieve detoxification activity , less environmental pollution, and less demanding environmental cleanliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of embodiment 1 Rhodococcus erythrococcus strain fermented liquid and supernatant thereof
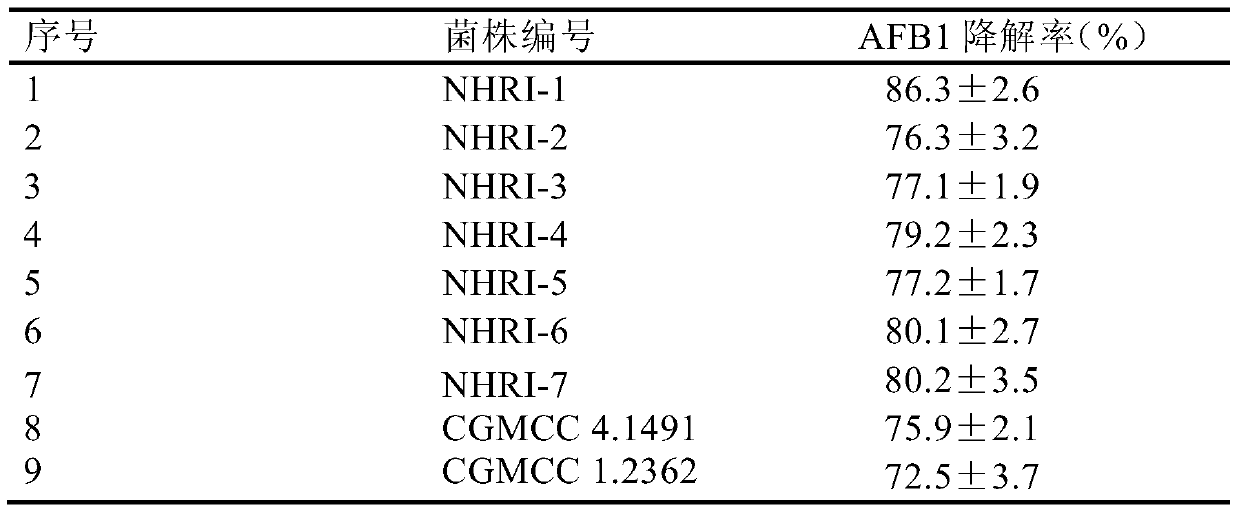
[0042] Seven strains of Rhodococcus erythropolis NHRI-1, NHRI-2, NHRI-3, NHRI-4, NHRI-5, NHRI-6 and NHRI-7 preserved in glycerol tubes at -80℃ General Microorganism Center (CGMCC), deposit numbers are CGMCC No.8590, CGMCC No.8591, CGMCC No.8592, CGMCC No.8593, CGMCC No.8594, CGMCC No.8595 and CGMCC No.8596) and purchased Add 50 μL of frozen storage solution of two strains of Rhodococcus erythrococcus CGMCC4.1491 and CGMCC1.2362 from the General Microbiology Center of China Microbiological Culture Collection Management Committee to 50mL liquid medium (composition: peptone 1.00%, beef extract 0.50%, glucose 0.50%, sodium chloride 0.50%; pH7.2-7.4, 121°C autoclave for 20min) activation culture, the culture conditions are: temperature 30°C, rotation speed 100r / min, culture time 48h. After confirming the purity of the corresponding activated culture solution by microscopi...
Embodiment 2
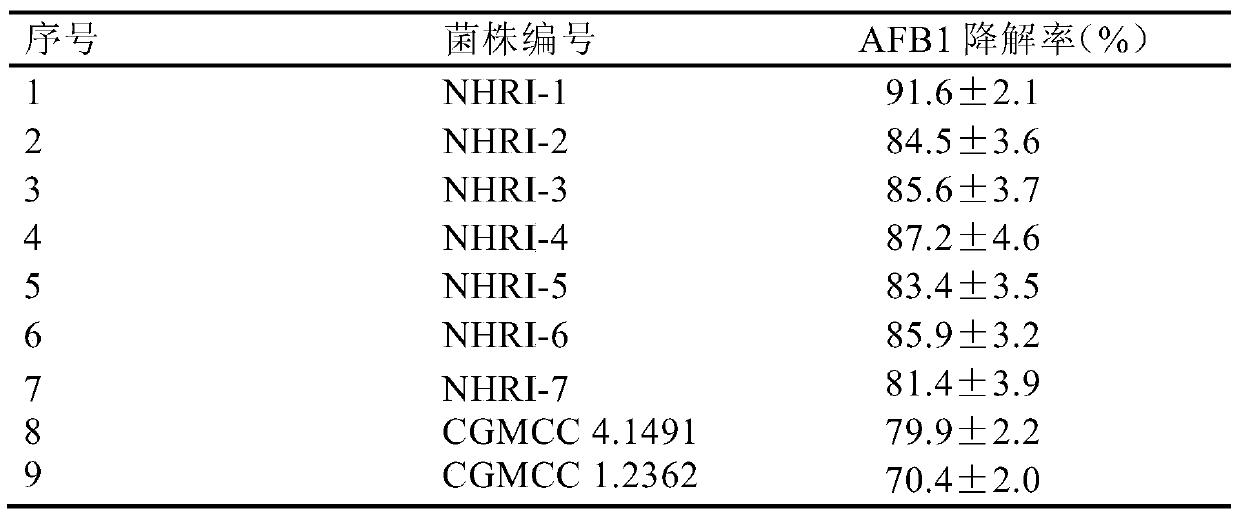
[0044] The degradation test of the Rhodococcus rhodococcus strain fermented liquid of embodiment 2 mixing ratio 3% to the AFB1 in the feed raw material
[0045] The fermentation broth of the strain preserved in Example 1 was evenly mixed into the feed material (corn, soybean, etc. cake mixture) polluted by AFB1 in a ratio of 3% (volume / weight ratio) (AFB1 content was 1mg / kg), each Prepare 3 mixed test samples from the fermentation broth of the strain, each sample 1000g, use sterile water to adjust the humidity to 60%-80%, put it in a 5L sterile Erlenmeyer flask, and react at a temperature of 30°C for 72h. During this process Keep the pH at 7.2-7.4. At the same time, under the same conditions, a 3% mixed sample of fermentation medium without Rhodococcus rhodococcus was set as a control.
[0046] After the reaction was completed, 5g of each sample was sampled, and the kit (MycoSep226Afazon + MultifunctionalColumns, Romer Labs Inc.MO, USA, production batch number: 226903-1306) ...
Embodiment 3
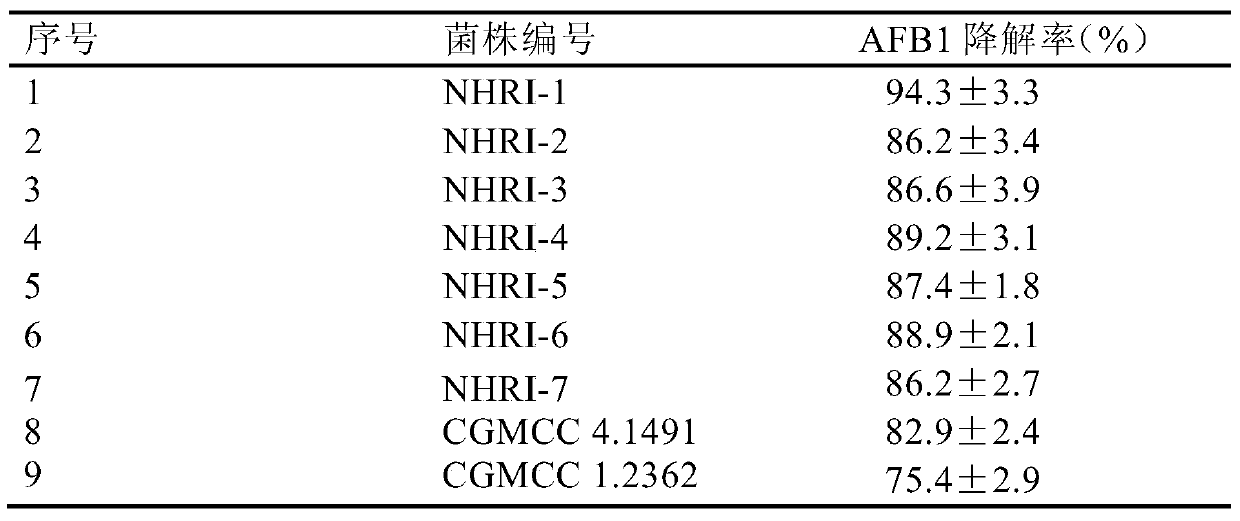
[0051] The degradation test of the Rhodococcus erythrococcus strain fermented liquid of embodiment 3 mixing ratio 8% to the AFB1 in the feed raw material
[0052] The fermentation broth of the strain preserved in Example 1 was evenly mixed into the feed material (corn, soybean, etc. cake mixture) polluted by AFB1 in a ratio of 8% (volume / weight ratio) (AFB1 content was 1mg / kg), each Prepare 3 mixed test samples from the fermentation broth of the strain, each sample 1000g, use sterile water to adjust the humidity to 60%-80%, put it in a 5L sterile Erlenmeyer flask, and react at a temperature of 30°C for 72h. During this process Keep the pH at 7.2-7.4. At the same time, under the same conditions, an 8% mixed sample of fermentation medium without Rhodococcus rhodococcus was set as a control.
[0053] After the reaction was completed, 5g of each sample was sampled, and the kit ((MycoSep226Afazon + Multifunctional Columns, Romer Labs Inc.MO, USA, production batch number: 226903-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com