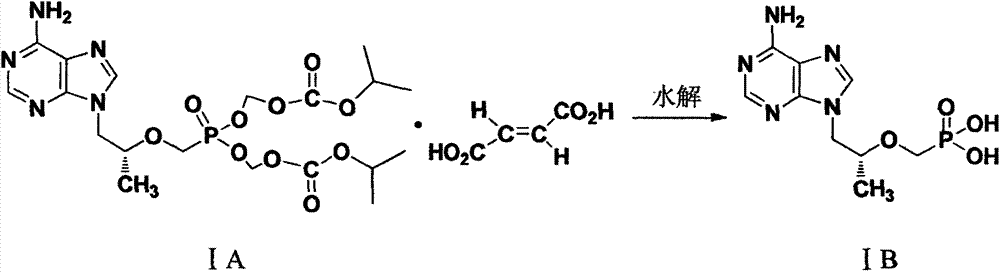
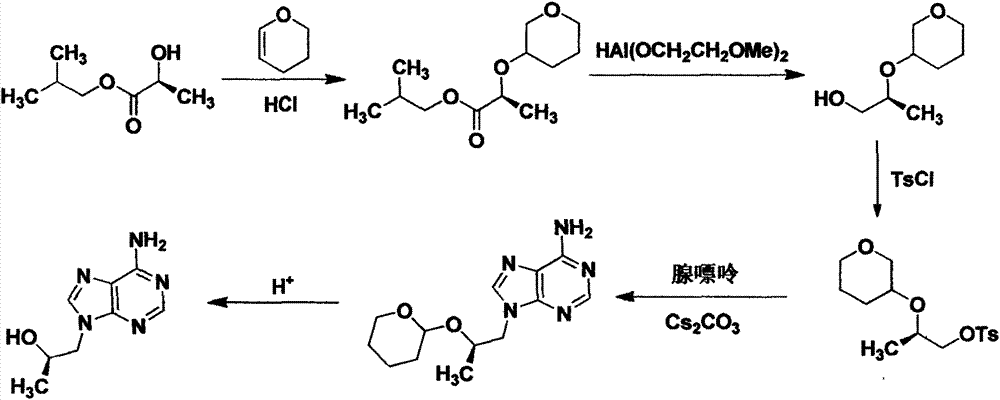
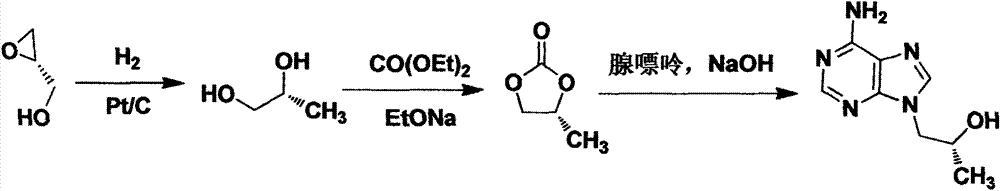
Preparation method of (R)-(+)-9-(2-hydroxypropyl) adenine
A technology of hydroxypropyl and adenine, which is applied in the field of --9-adenine and its preparation, and can solve problems such as limiting industrial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 1mmol of 6-chloropurine and 3mmol of potassium carbonate successively to 6mL of DMF (dimethylformamide, the same below), stir for 10 minutes under ice-bath conditions, add bromoacetone 0.168mL (2mmol), and react for 1 hour under ice-bath. Add appropriate amount of water, extract 3 to 5 times with ethyl acetate, dry the organic phase with anhydrous sodium sulfate, and separate by column chromatography to obtain 6-chloro-9-(acetonyl)purine. Yield 96%.
[0036] Add 5 mol% prolinol ligand and 1 mol% ruthenium catalyst into 0.5 mL acetonitrile, stir at room temperature for 1 hour, then add HCOONa·2H 2 O (1mmol), after stirring for ten minutes, add 0.1mol of 6-chloro-9-(acetonyl)purine, react at room temperature for 24 hours, separate and purify by column chromatography to obtain 6-chloro-9-(acetonyl)purine, produce Yield 12%, enantioselectivity 98%.
[0037] At 0°C, add 0.5mmol of 6-chloro-9-(acetonyl)purine into 50ml of newly prepared ammonia methanol solution, react ...
Embodiment 2
[0039] Add 1 mmol of 6-chloropurine and 3 mmol of potassium carbonate to 6 mL of DMF in sequence, stir for 10 minutes under ice-bath conditions, add bromoacetone 0.168 mL (2 mmol), react for 1 hour under ice-cooling, add appropriate amount of water, and use ethyl acetate Extract 3 to 5 times, dry the organic phase with anhydrous sodium sulfate, and separate by column chromatography to obtain 6-chloro-9-(acetonyl)purine. Yield 96%.
[0040] Add 5 mol% prolinol ligand and 5 mol% ruthenium catalyst into 0.5 mL acetonitrile, stir at room temperature for 1 hour, then add HCOONa·2H 2 O (1mmol), after stirring for ten minutes, add 0.1mol of 6-chloro-9-(acetonyl)purine, room temperature for 24 hours, column chromatography separation, get 6-chloro-9-(acetonyl)purine, yield 99%, enantioselectivity 98%.
[0041] At 0°C, add 0.5mmol of 6-chloro-9-(acetonyl)purine into 50ml of newly prepared ammonia methanol solution, react at 60°C for 48 hours, and separate by column chromatography to o...
Embodiment 3
[0043] Add 1 mmol of 6-chloropurine and 3 mmol of potassium carbonate to 6 mL of DMF in sequence, stir for 10 minutes under ice-bath conditions, add bromoacetone 0.168 mL (2 mmol), react for 1 hour under ice-cooling, add appropriate amount of water, and use ethyl acetate Extract 3 to 5 times, dry the organic phase with anhydrous sodium sulfate, and separate by column chromatography to obtain 6-chloro-9-(acetonyl)purine. Yield 96%.
[0044] Add 2 mol% prolinol ligand and 5 mol% ruthenium catalyst into 0.5 mL acetonitrile, stir at room temperature for 1 hour, then add HCOONa·2H 2 O (1mmol), after stirring for ten minutes, add 0.1mol of 6-chloro-9-(acetonyl)purine, room temperature for 24 hours, column chromatography separation, get 6-chloro-9-(acetonyl)purine, yield 95%, enantioselectivity 45%.
[0045] At 0°C, add 0.5mmol of 6-chloro-9-(acetonyl)purine into 50ml of newly prepared ammonia methanol solution, react at 60°C for 48 hours, and separate by column chromatography to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com