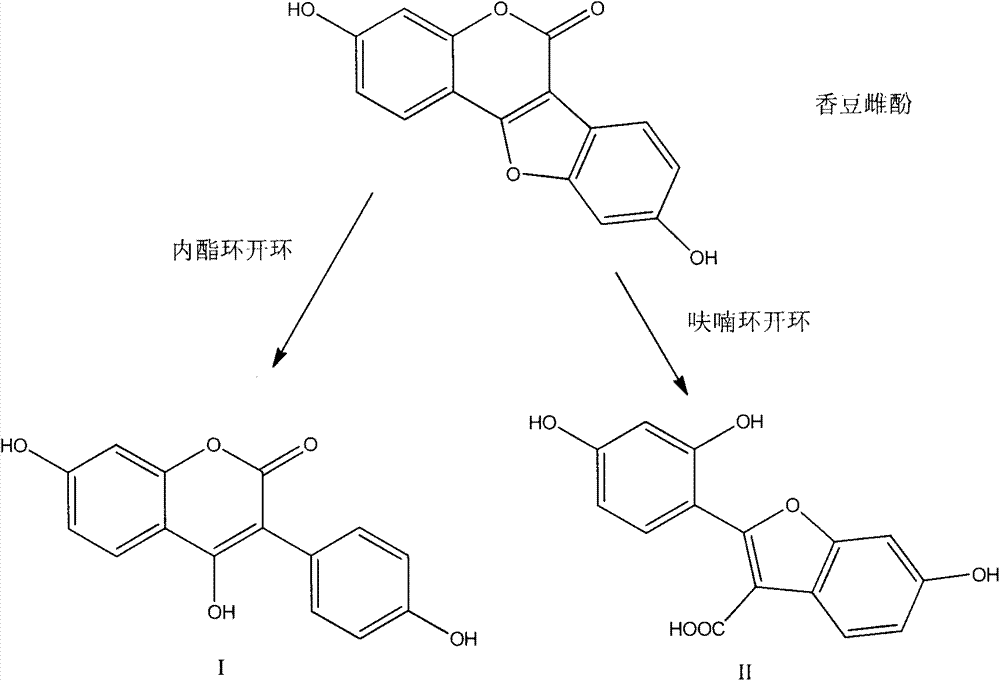
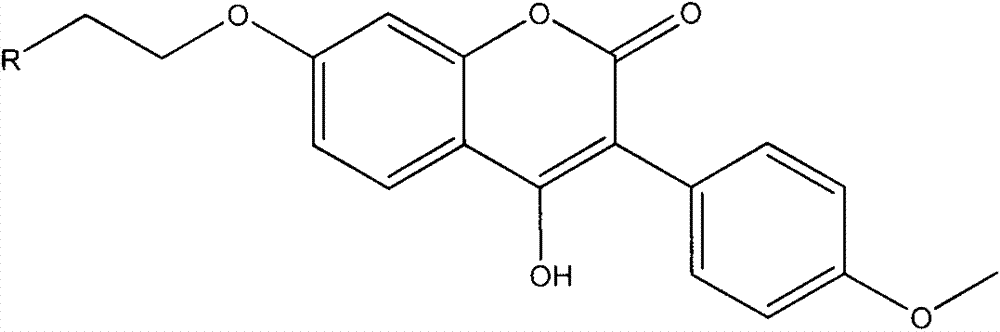
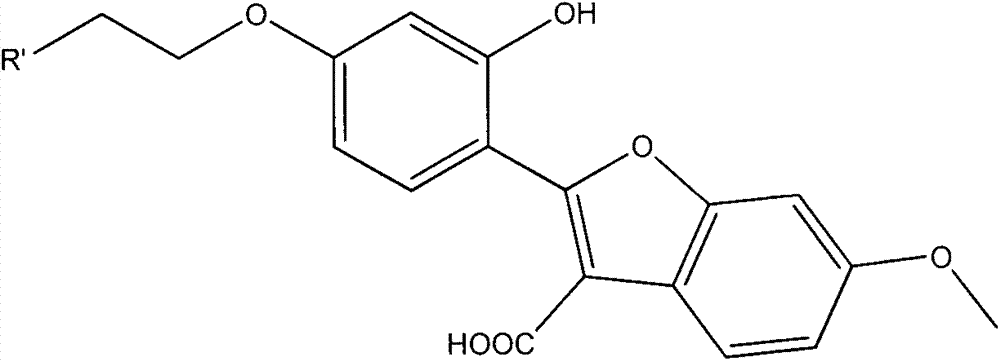
Coumestrol open-ring analogue and medical application thereof
A pharmacy and drug technology, which is applied to the ring-opening analog of coumestrol to prepare the field of drugs as angiogenesis inhibitor and vascular blocker, and can solve the problems of no very satisfactory treatment method, low toxicity and high efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 1-(2,4-dihydroxyphenyl)-2-(4-methoxyphenyl)ethanone (11)
[0033] Dissolve 3.60 g (32.5 mmol) of resorcinol in 50 ml of newly steamed boron trifluoride ether solution, and then add 5.00 g (30.0 mmol) of p-methoxyphenylacetic acid. The mixture was reacted at 100°C, the end point was determined by TLC, washed and extracted three times with saturated sodium bicarbonate solution, and the organic phase was dried overnight by adding anhydrous sodium sulfate. Concentrate under reduced pressure to remove the solvent, and recrystallize the crude product from absolute ethanol to obtain 5.95 g of off-white powder with a yield of 77%, mp150-152 °C, EI-MS m / z: 259 [M+H] + .
Embodiment 2
[0035] Synthesis of 4,7-dihydroxy-3-(4-methoxyphenyl)-coumarin (12)
[0036] Add 4.4 g of compound 11 (17.05 mmol) and 8.2 g of NaH (341 mmol) into 120 ml of diethyl carbonate, react at 80° C. under nitrogen protection, and determine the end point by TLC. Add 100ml of water into the reaction liquid, separate the liquids, discard the organic phase, extract the water phase with ethyl acetate three times, adjust the pH of the water phase to 3-4 with 1mol / L hydrochloric acid, a large amount of white solid precipitates out, and suction filter to obtain the crude product , after purification by column chromatography, 3.8g white powder was obtained, the yield was 80%, ESI-MS m / z: 283[M-H] - .
Embodiment 3
[0038] Synthesis of N-Chloroethylpyrrolidine Hydrochloride
[0039] 4.6ml of pyrrolidine (57.5mmol) and 4.6ml of 2-chloroethanol (69.0mmol) were added to 40ml of toluene, heated to reflux for 2 hours, the reaction solution was cooled to room temperature, and 8.4ml of thionyl chloride (114mmol ), after 20 minutes of dripping, the ice bath was removed, stirred at room temperature for 6 hours, the toluene and thionyl chloride were evaporated under reduced pressure, and the residual solid was recrystallized with ethanol to obtain 8.3 g of chloroethylpyrrolidine hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com