Method for establishing acute viral hepatitis animal model
A viral hepatitis and animal model technology, applied in the fields of liver immunology and experimental zoology, can solve the problems of poor effect of acute liver injury and achieve good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
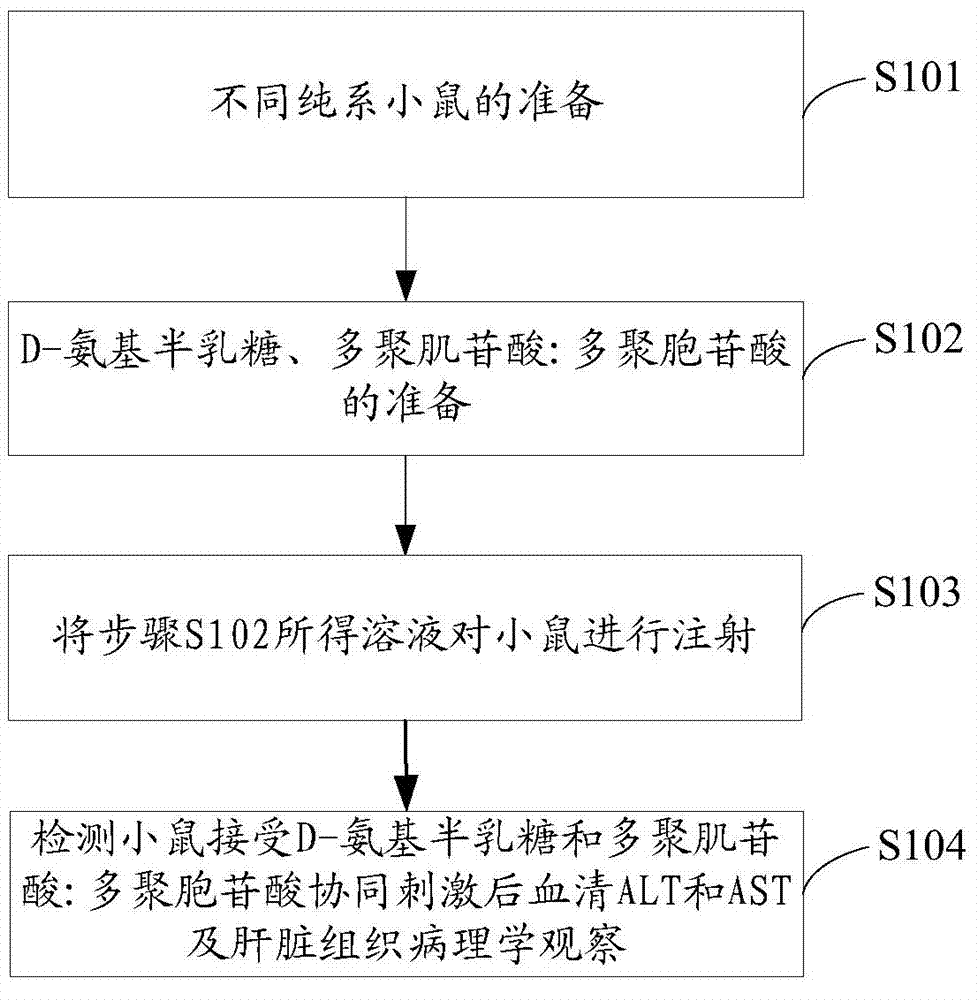
[0032] Embodiment 1 of the present invention provides a method for establishing an animal model of acute viral hepatitis, such as figure 1 shown, including the following steps:
[0033] Step S101: Preparation of different pure strains of mice:
[0034] From pure-line white mice (pure-line BALB / c mice), pure-line B6 mice (pure-line C57BL / 6J mice), pure-line severe combined immunodeficiency (SCID) mice, pure-line nude mice (pure-line C57BL / 6J mice) At least one group of mice was selected from nude mice with BALB / c background) or pure line differentiation antigen 1d (CD) deficient mice (mice with pure line BALB / c background), wherein the age of mice was 6-8 weeks Zhou, the animal model established by mice of this age has high symptom stability, and it is relatively easy to simulate human diseases. The following experiments all use female mice. The mice are kept in a pathogen-free animal room, and the feed and water are sterilized. Prevent viral infection.
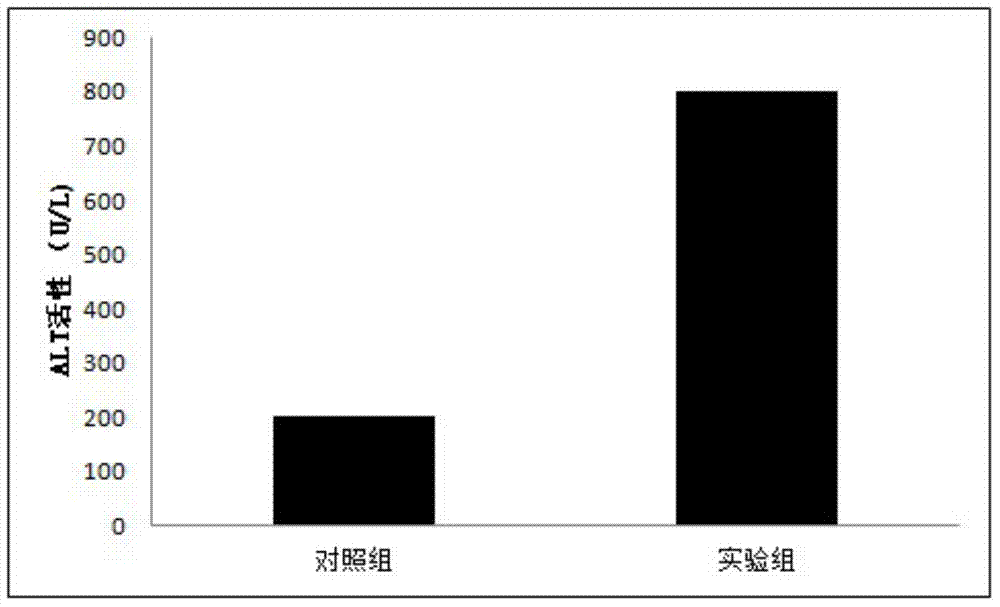
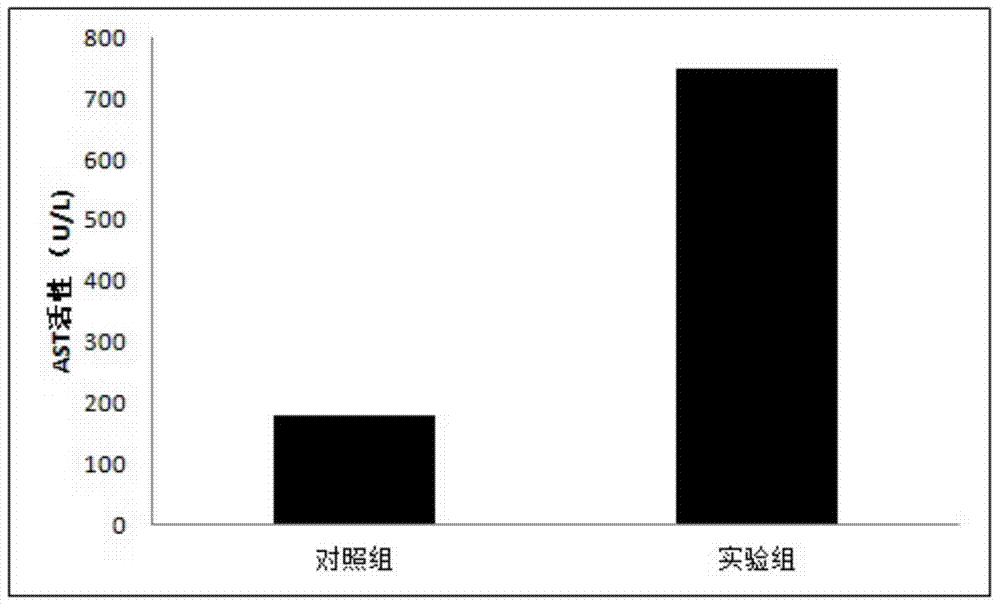
[0035] Step S102: D-g...
Embodiment 2
[0047] Embodiment 2 of the present invention provides a method for establishing an animal model of acute viral hepatitis, comprising the following steps:
[0048] Step S201: Preparation of different pure strains of mice:
[0049] Pure-line white mice (pure-line BALB / c mice) were selected, aged 6-8 weeks, female. The mice were kept in a pathogen-free animal room, and the feed and water were sterilized to prevent virus infection.
[0050] Step S202: D-galactosamine, polyinosinic acid: preparation of polycytidylic acid:
[0051] Prepare D-galactosamine solution with sterile phosphate buffer solution (PBS solution), prepare polyinosinic acid:polycytidylic acid solution with sterile phosphate buffer solution, wherein, the final concentration of D-galactosamine solution is respectively 20 μg / μl, polyinosinic acid: polycytidylic acid solution final concentration is 10ng / μl, D-galactosamine and polyinosinic acid: polycytidylic acid are all purchased from Sigama Company in the United ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com