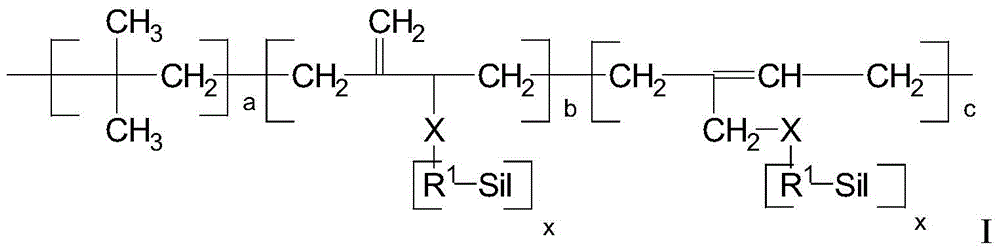
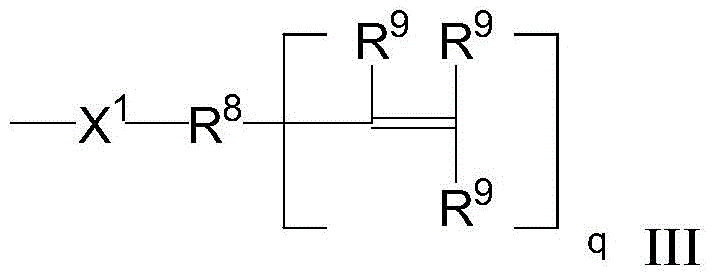
Functionalized isobutylene-isoprene copolymer compositions
A composition and copolymer technology, applied in the direction of conjugated diene coatings, surgical adhesives, dressings, etc., can solve problems such as difficult to split uniform, homogeneous coating, incompatibility, and reduce the physical properties of the coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0096] abbreviation:
[0097] ●PDMS – polydimethylsiloxane
[0098] ●BPIB – brominated polyisobutylene (co)polymer
[0099] Table 1: Materials
[0100]
[0101] Reference Example 1: Preparation of Polymer A
[0102] In a three necked round bottom flask equipped with reflux condenser, thermometer and nitrogen inlet were placed 12.2 g of BPIB, 0.61 g of UDA, 0.22 g of TBAB and 48.8 g of toluene. The contents of the flask were stirred with a magnetic stir bar at room temperature under nitrogen atmosphere. After all components were completely dissolved, the flask was heated to 105°C. After 3 hours, the reaction was cooled to room temperature. The solution was poured into acetone to coagulate the modified polymer. The isolated polymer was washed 3 times with fresh acetone to remove residual UDA and TBAB. Polymer A (10-undecylenic acid grafted PIB) was then filtered and dried in a vacuum oven at 50°C for 12 hours.
[0103] Reference Example 2: Preparation of Polymer...
example 1
[0106] In a three necked round bottom flask equipped with reflux condenser, thermometer and nitrogen inlet were placed 5.0 g of polymer A, 2.0 g of PDMS1, 0.05 g of catalyst and 20.0 g of toluene. The contents of the flask were stirred with a magnetic stir bar at 60°C under nitrogen atmosphere. After 6 hours, the reaction was cooled to room temperature and the solution was poured into acetone to condense the PDMS-grafted polymer. The isolated polymer was washed 3 times with fresh acetone to remove unreacted PDMS and catalyst. The PDMS-grafted PIB polymer was then dried in a vacuum oven at 50 °C for 12 h. Based on NMR analysis, the PDMS-grafted PIB polymer contained 3.2 wt% PDMS.
example 2
[0108] The PDMS-grafted PIB polymer was prepared according to the procedure of Example 1, except that the starting materials were 10.0 g of polymer A, 5.0 g of PDMS2, 0.067 g of catalyst and 40 g of toluene, and the reaction of the flask was magnetically A stir bar was stirred at 60 °C under nitrogen for 24 hours. Based on NMR analysis, the PDMS-grafted PIB polymer contained 21.1 wt% PDMS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com