A Method for Controlling the Domain Orientation of Liquid Crystal Polymers via the Anchor Effect of Surface Coatings
A liquid crystal polymer and surface coating technology, applied in the field of materials, can solve the problem of uncontrollable domain orientation of homopolymers, and achieve the effects of excellent solubility and film-forming properties, large polarizability, and easy access.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A liquid crystal polymer, its structural formula is:
[0040]
[0041] Raw materials: p-butylaniline, 1,10-dibromodecane, potassium nitrite, hydrochloric acid, sodium hydroxide, methacrylic acid, N,N-dimethylformamide (DMF), tetrahydrofuran (THF), phenol, Petroleum ether, chlorobenzene, polyvinylpyrrolidone.
[0042] Liquid crystal monomer; 10-[4-(4-phenoxy)-p-butylazobenzene]decyl methacrylate
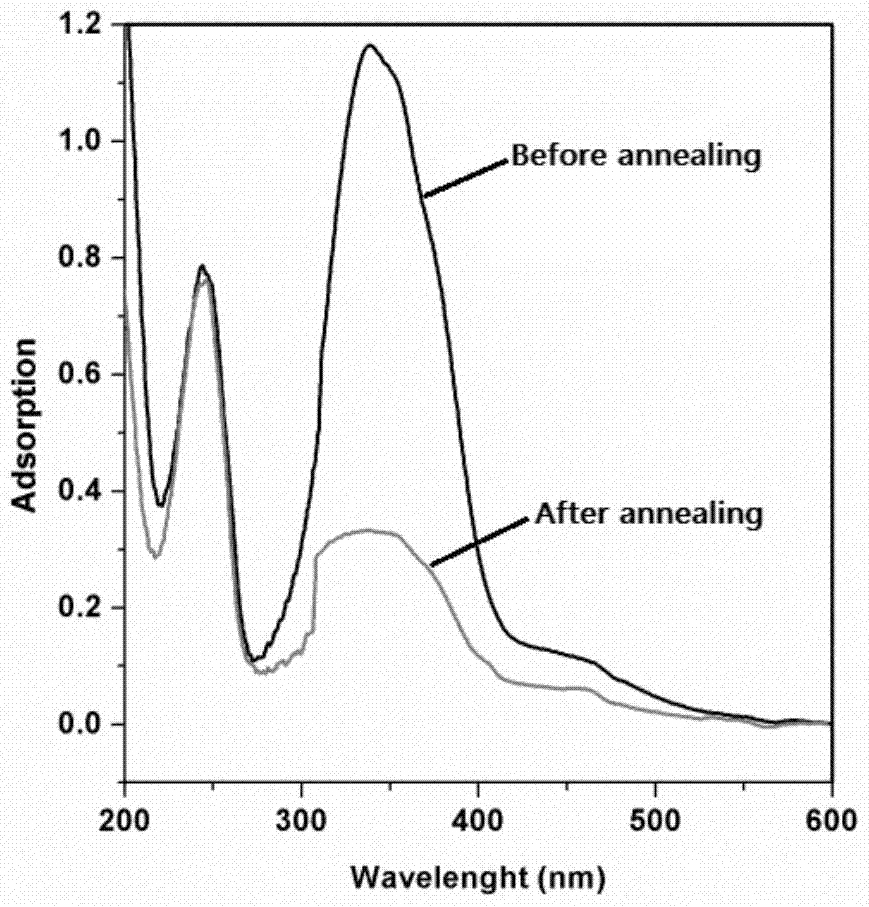
[0043] Firstly, the liquid crystal monomer is synthesized by free radical polymerization into a liquid crystal polymer, and the liquid crystal polymer is dissolved in chlorobenzene, and the liquid crystal polymer film is obtained by spin coating, and its thickness is about 100-200nm. In order to facilitate the comparison effect, After solvent volatilization, a part of the liquid crystal polymer film was annealed at 130°C for 10 minutes and then lowered to room temperature, and then the alignment of the liquid crystal polymer domains before and after annealing without coveri...
Embodiment 2
[0046] A liquid crystal polymer, its structural formula is:
[0047]
[0048] Raw materials: p-butylaniline, 1,10-dibromodecane, potassium nitrite, hydrochloric acid, sodium hydroxide, methacrylic acid, N,N-dimethylformamide (DMF), tetrahydrofuran (THF), phenol, Petroleum ether, chlorobenzene, polyacrylamide.
[0049] Liquid crystal monomer; 10-[4-(4-phenoxy)-p-butylazobenzene]decyl methacrylate
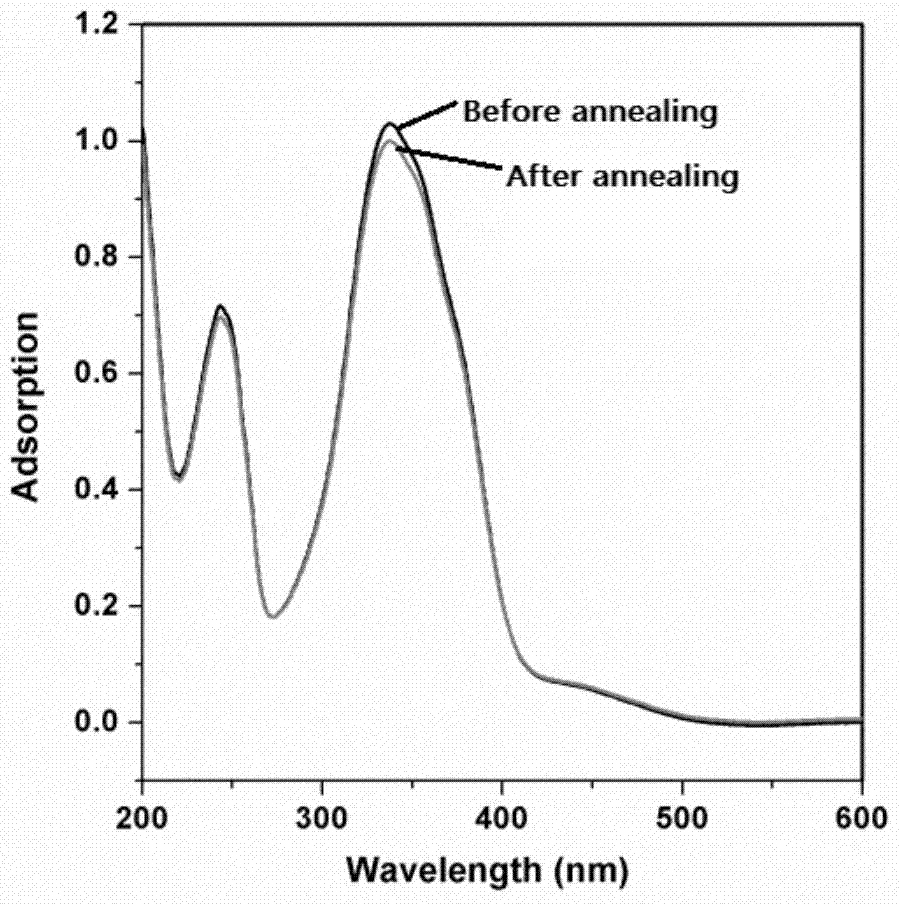
[0050] Firstly, the liquid crystal monomer is synthesized by free radical polymerization into a liquid crystal polymer, and the liquid crystal polymer is dissolved in chlorobenzene, and the liquid crystal polymer film is obtained by spin coating, and its thickness is about 100-200nm. In order to facilitate the comparison effect, After solvent volatilization, a part of the liquid crystal polymer film was annealed at 130°C for 10 minutes and then lowered to room temperature, and then the alignment of the liquid crystal polymer domains before and after annealing without covering the...
Embodiment 3
[0053] A liquid crystal polymer, its structural formula is:
[0054]
[0055] Raw materials: p-butylaniline, 1,10-dibromodecane, potassium nitrite, hydrochloric acid, sodium hydroxide, methacrylic acid, N,N-dimethylformamide (DMF), tetrahydrofuran (THF), phenol, Petroleum ether, chlorobenzene, polymethylmethacrylate-methacrylic acid.
[0056] Liquid crystal monomer; 10-[4-(4-phenoxy)-p-butylazobenzene]decyl methacrylate
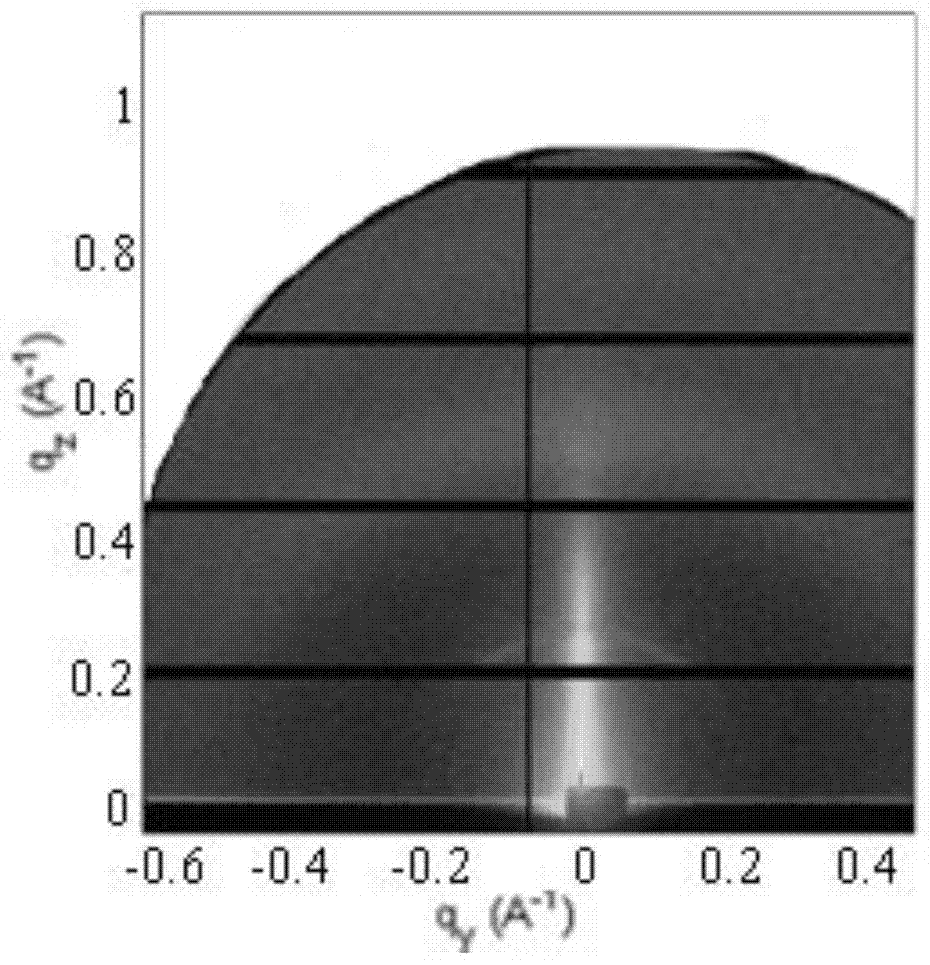
[0057] Firstly, the liquid crystal monomer is synthesized by free radical polymerization into a liquid crystal polymer, and the liquid crystal polymer is dissolved in chlorobenzene, and the liquid crystal polymer film is obtained by spin coating, and its thickness is about 100-200nm. In order to facilitate the comparison effect, After solvent volatilization, a part of the liquid crystal polymer film was annealed at 130°C for 10 minutes and then lowered to room temperature, and then the alignment of the liquid crystal polymer domains before and after annea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com