Terminal-modified conjugated diene-vinyl aromatic copolymer and its synthesis method
A technology based on aromatic hydrocarbon copolymers and vinyl aromatic hydrocarbons, applied in the field of conjugated diene-vinyl aromatic hydrocarbon copolymers and their synthesis, can solve the problems of difficult dispersion of carbon black and achieve excellent rolling resistance and good compatibility performance, excellent wet skid resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] According to an embodiment of the present invention, the synthesis method of the terminal-modified conjugated diene-vinyl aromatic copolymer comprises the following steps:
[0023] (a) reacting a conjugated diene monomer with a vinyl aromatic hydrocarbon monomer to produce a conjugated diene-vinyl aromatic hydrocarbon copolymer;
[0024] (b) reacting the conjugated diene-vinyl aromatic hydrocarbon copolymer with the first modifier to generate an intermediate product; and
[0025] (c) reacting the intermediate product with a second modifying agent to produce an end-modified conjugated diene-vinyl aromatic hydrocarbon copolymer.
[0026] The above steps are described in detail below:
[0027] step (a)
[0028] Step (a) may be carried out by any known method for polymerizing conjugated diene monomers and vinyl aromatic hydrocarbon monomers. For example, a conjugated diene monomer and a vinyl aromatic hydrocarbon monomer may be polymerized in the presence of a polymeriza...
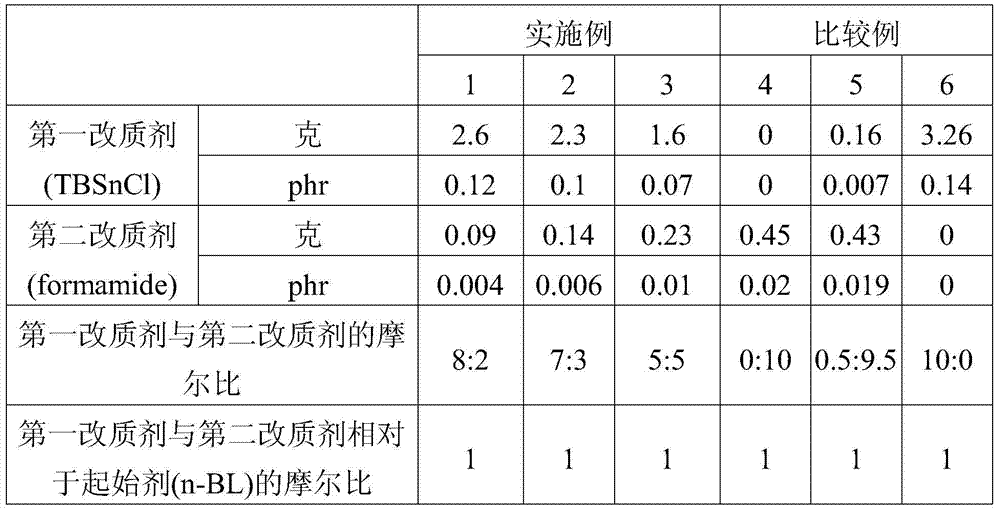
Embodiment 1
[0081] First, 8 kg of cyclohexane was added into the reaction tank as a solvent, and the temperature of the reaction system was maintained at 45°C. Next, 6.4 grams of 2,2-bis(2-tetrahydrofuryl)propane (2,2-di(2-tetrahydrofuryl)propane, DTHFP) was added into the reaction tank as a microstructure modifier. Then, 0.8 g of n-butyllithium (n-BL) was added into the reaction tank as an initiator for the polymerization reaction. Here, the molar ratio of the microstructure modifier to the polymerization initiator is substantially about 2:1. Next, take 447 grams of styrene (styrene) as the vinyl aromatic hydrocarbon monomer, and 1683 grams of butadiene (butadiene) as the conjugated diene monomer, and put them into the reaction tank for polymerization. After that, 74.6 g of butadiene monomer was added for reaction. At this time, after sampling and removing the solvent of the sample, the content of the 1,2 structure in the copolymer was measured by IR or NMR, and it was found that the p...
Embodiment 2-3
[0084] Embodiment 2-3 and comparative example 4-6
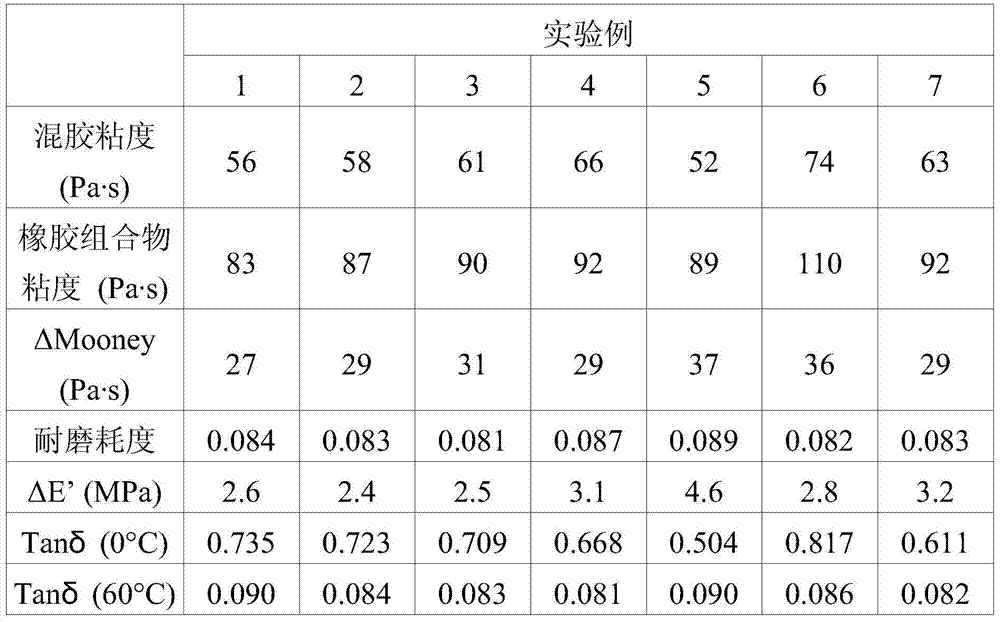
[0085] The terminal-modified conjugated diene-vinyl aromatic hydrocarbon copolymers of Examples 2-3 and Comparative Examples 4-6 were prepared in the same steps as Example 1. However, the difference lies in the amount of the first modifying agent and the second modifying agent (as shown in Table 1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com