Synthesis method of high-purity high-yield syringic acid
A synthesis method and a high-yield technology, applied in the field of high-purity and high-yield syringic acid synthesis, can solve problems such as poor appearance quality, low product purity, immature cost and technology, and achieve reduced synthesis cost and high purity. , easy manipulation and industrialized effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of synthetic method of high purity, high yield syringic acid, comprises the following steps:
[0032] (1) Esterification:
[0033] Put 18.2g (0.1mol) of syringaldehyde (3,5-dimethoxy-4-hydroxybenzaldehyde) and 25.5g (0.25mol) of acetic anhydride in a 50ml reaction flask, stir and heat up to reflux for 2 hours, then cool down to 35°C;
[0034] (2) Oxidation:
[0035] Continuously and evenly drop 24.2g (0.2mol) of 28% hydrogen peroxide into the esterification reaction solution in step (1) within 0.5 hours, then raise the temperature to 55°C, keep it warm for 10 hours, cool down to 5°C, filter with suction, wash with water Cake to pH=7;
[0036] (3) Hydrolysis:
[0037] Put the filter cake into a 100ml reaction bottle, add 48g of 10% sodium hydroxide aqueous solution, heat up to 80°C, keep stirring for 3 hours, add 98% concentrated sulfuric acid dropwise to pH = 2, cool the system to 25°C, and filter with suction , wash the filter cake with water to pH=7, and t...
Embodiment 2
[0040] (1) Esterification:
[0041] Put 728g (4.0mol) of syringaldehyde, 1020g (10.0mol) of acetic anhydride, and 800g of acetic acid into a 2000ml reaction flask, stir and heat up to reflux for 3 hours, then cool down to 30°C;
[0042] (2) Oxidation:
[0043] Continuously and evenly drop 680g (5.6mol) of 28% hydrogen peroxide into the esterification reaction solution in step (1) within 2 hours, then raise the temperature to 60°C, keep it warm for 14 hours, cool down to 3°C, filter with suction, and wash the filter cake with water to pH=7;
[0044] (3) Hydrolysis:
[0045] Put the filter cake into a 3000ml reaction bottle, add 2080g of 10% sodium hydroxide aqueous solution, heat up to 110°C, keep stirring for 1.5 hours, then add 98% concentrated sulfuric acid dropwise to pH = 2, cool the system to 20°C, and filter with suction , wash the filter cake with water to pH = 7, and the resulting off-white product is syringic acid.
[0046] The prepared syringic acid has a good ap...
Embodiment 3
[0048] According to the step of embodiment 1, change each process parameter, test:
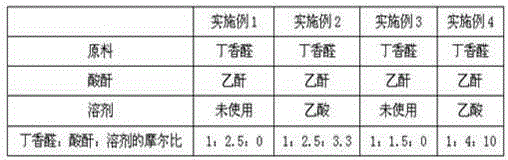
[0049] Table 1 Raw material ratio
[0050]
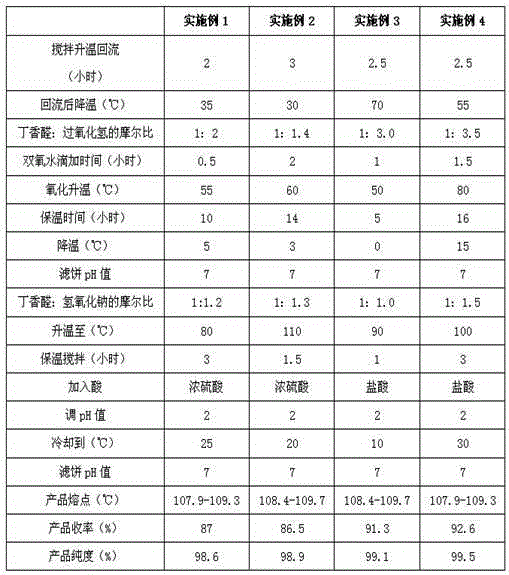
[0051] Table 2 The process parameters of the present invention's synthetic syringic acid
[0052]
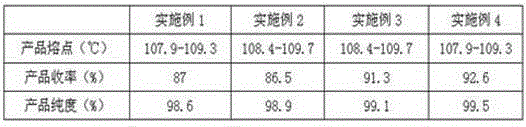
[0053] Table 3 The product index prepared by the present invention
[0054]
[0055] Tested:
[0056] The acid anhydride is acetic anhydride, phthalic anhydride, preferably acetic anhydride.
[0057] Described solvent is acetic acid, acetone, toluene, dichloroethane, preferably acetic acid.
[0058] Described mineral acid is hydrochloric acid, sulfuric acid, preferably sulfuric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com