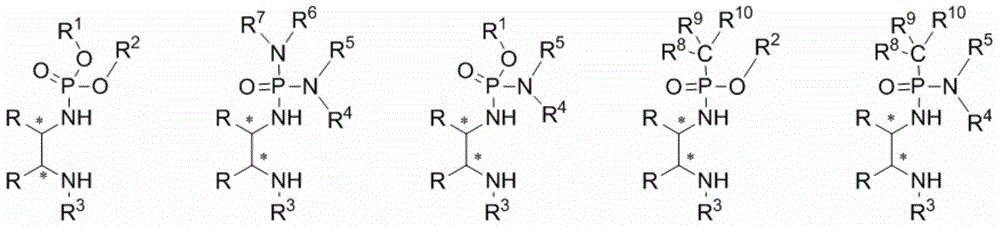
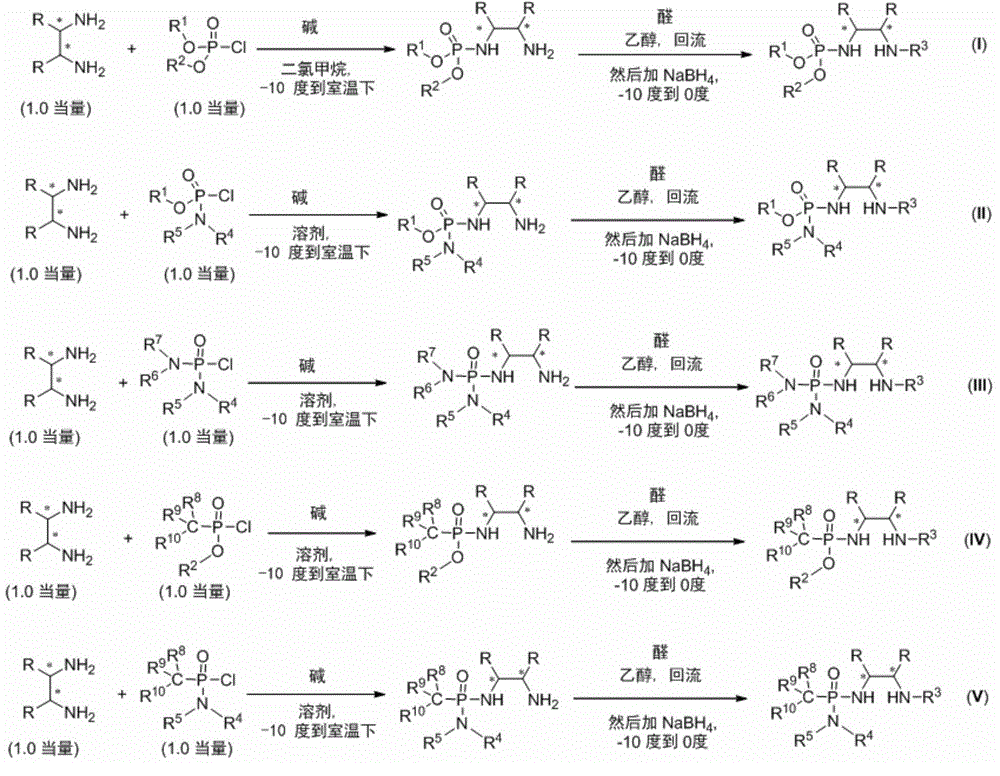
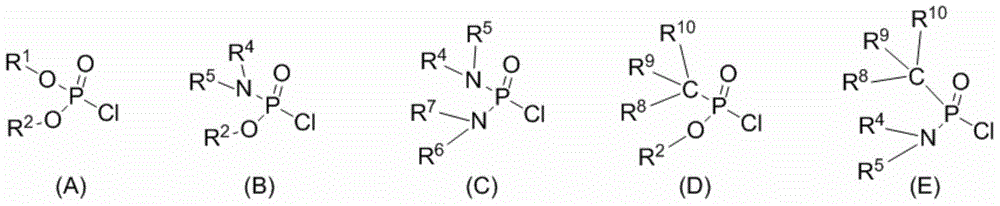
Phosphamide-(di) secondary amine dual-functional catalyst and synthesis method thereof
A technology of bifunctional catalysts and synthesis methods, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc. , chiral bifunctional catalyst application reports and other issues, to achieve the effect of simple and convenient operation, stable properties, and new structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Under nitrogen protection, add cinchonidine-derived primary amine Ia (1.0 equiv) and anhydrous CH to a 50 mL round bottom flask 2 Cl 2 , after complete dissolution, triethylamine (2.0 equiv) was added. Diethoxyphosphoryl chloride IIa (1.2 equiv) was added dropwise at 0°C, stirred overnight and post-treated: add 30mL of water, extract with dichloromethane, dry over anhydrous sodium sulfate and column chromatography to obtain a white solid product IIIa, 80% yield. 1 H NMR (400MHz, CDCl 3 ):δ4.10-4.04(m,4H),2.63-2.56(m,2H),2.29-2.28(m,1H),2.05-2.02(m,1H),1.94-1.91(m,1H),1.67 (s,br,2H),1.59(s,2H),1.31(t,J=6.8Hz,6H),1.25-1.05(m,4H); 13 C NMR (100MHz, CDCl 3 ):δ62.46,62.42,58.89,56.66(d,J C-P =6.5Hz,1C),34.85,34.67,25.37,24.92,16.26,16.19; 31 P NMR (161.7MHz, CDCl 3 ):δ9.03(s,1P).MS(EI):250(M + ,0.4),154(25),126(13),97(100),96(22),44(39);HRMS(EI):Exact mass calcd for C 10 h 23 N 2 o 3 P[M] + :250.1446,Found:250.1445.
Embodiment 2
[0043]
[0044] Under nitrogen protection, add cinchonidine-derived primary amine Ia (1.0 equiv) and anhydrous CH to a 50 mL round bottom flask 2 Cl 2 , after complete dissolution, triethylamine (2.0 equiv) was added. Diethoxyphosphoryl chloride IIa (1.0 equiv) was added dropwise at 0°C, stirred overnight and post-treated: add 30mL of water, extract with dichloromethane, dry over anhydrous sodium sulfate and perform column chromatography to obtain a white solid product IIIa, 80% yield. Then, under nitrogen protection, add anhydrous EtOH after adding IIIa in the Schlenk bottle, add 1-naphthaldehyde (1.2equiv) at room temperature, after the reaction system was stirred for 1 hour under reflux conditions (TLC detects that the reaction is complete), the The reaction system was transferred to an ice-water bath for cooling, then sodium borohydride (2.0 equivs) was slowly added, stirred in an ice-water bath for about 2 hours, and the reaction was complete, and NaHCO was added 3 ...
Embodiment 3
[0046]
[0047] Under nitrogen protection, add cinchonidine-derived primary amine Ia (1.0 equiv) and anhydrous CH to a 50 mL round bottom flask 2 Cl 2 , after complete dissolution, triethylamine (2.0 equiv) was added. Add diisopropoxyphosphoryl chloride IIb (1.0 equiv) dropwise at 0°C, stir overnight, and then perform post-treatment: add 30 mL of water, extract with dichloromethane, dry over anhydrous sodium sulfate, and column chromatography to obtain a white solid Product IIIb, yield 57%. Then under nitrogen protection, add anhydrous EtOH after adding IIIb in the Schlenk bottle, add 1-naphthaldehyde (1.2equiv) at room temperature, and the reaction system is stirred under reflux conditions after 1 hour (TLC detects that the reaction is complete), and The reaction system was transferred to an ice-water bath for cooling, then sodium borohydride (2.0 equivs) was slowly added, stirred in an ice-water bath for about 2 hours, and the reaction was complete, and NaHCO was added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com