Nickel-cobalt oxide electrode material as well as preparation method and applications thereof
A technology of electrode materials and oxides, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of high electrolyte selectivity, poor high-current discharge performance, and low theoretical capacity of graphite materials, achieving excellent charge and discharge performance, The effect of simple process and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
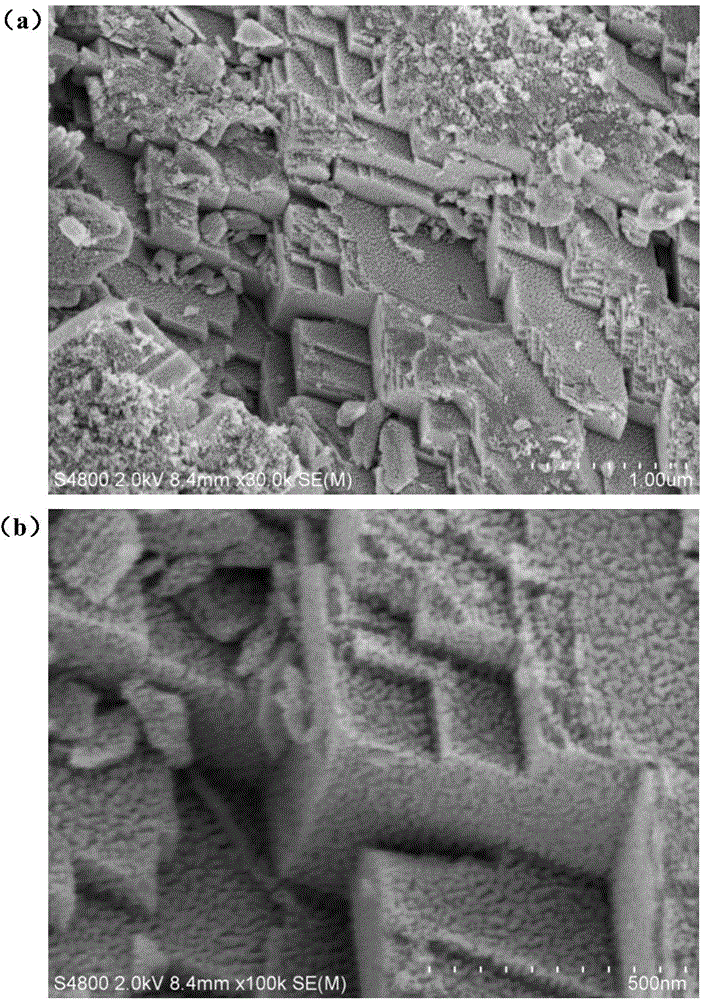
[0030] 50mmol of Ni(NO 3 ) 2 ·6H 2 O and 50mmol of Co(NO 3 ) 2 ·6H 2 O mix well, add 80mL distilled water, fully stir for 1 hour, then add 30mL methanol and 1g polyvinylpyrrolidone (PVP), fully stir for 1 hour; then add 20g of urea, mix well and pour into 100mL reaction kettle, Keep at 120°C for 10 hours; after the reaction, cool down to room temperature naturally, wash with a large amount of distilled water, dry in a blast drying oven at 80°C to form a solid powder, put the powder into a muffle furnace, and heat at a speed of 5°C / min to 350°C, and then keep it warm for 2 hours to obtain the nickel-cobalt oxide electrode material. The electrode material topography figure that embodiment 1 prepares is as follows figure 1 (a) and figure 1 (b) shown.
Embodiment 2
[0032] 150mmol of Ni(NO 3 ) 2 ·6H 2 O and 150mmol of Co(NO 3 ) 2 ·6H 2 O mix well, add 80mL distilled water, stir well for 1 hour, then add 80mL methanol and 5g polyvinylpyrrolidone, stir well for 1 hour; then add 30g urea, mix well, pour into 100mL reaction kettle, keep at 180°C for 24 Hours; after the reaction, cool down to room temperature naturally, wash with a large amount of distilled water, and dry it into a solid powder in a blast drying oven at 80°C, put the powder into a muffle furnace, and heat up to 350 ℃, and then keep it warm for 2 hours to obtain the nickel-cobalt oxide electrode material.
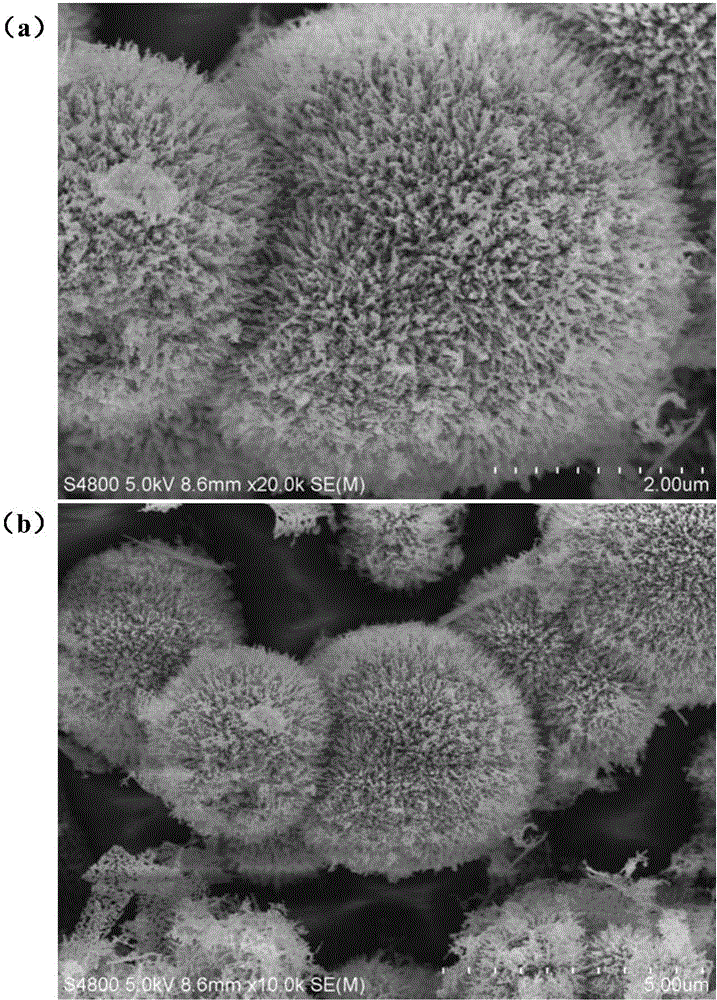
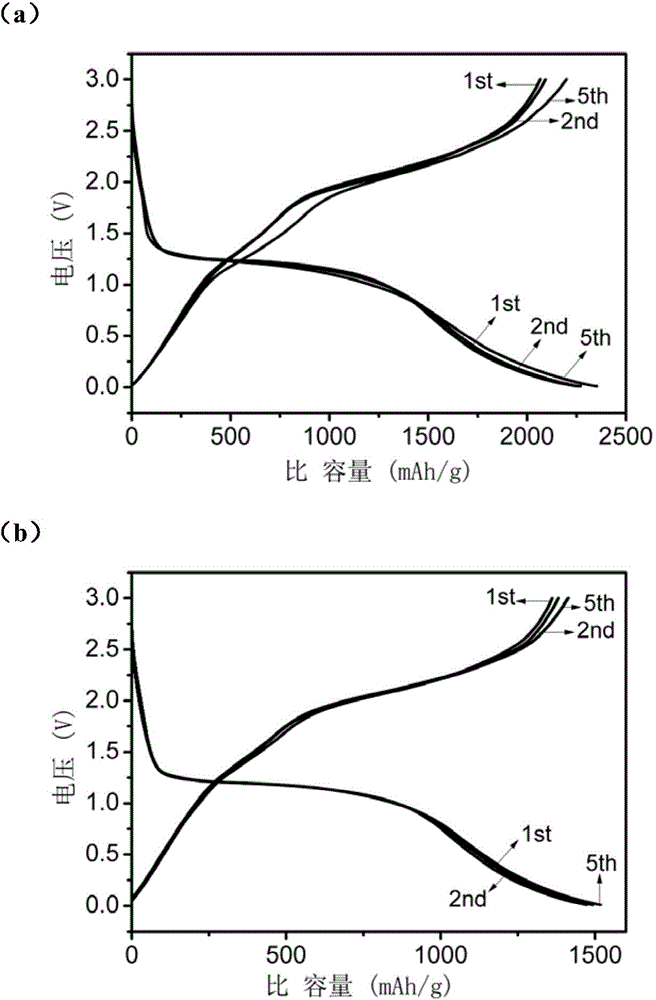
[0033] The electrode material topography figure that embodiment 2 prepares is as figure 2 (a) and figure 2 (b) shown. After being made into a battery, the first three charge and discharge data of the material are as follows: image 3 (a) and image 3 (b) shown. image 3 (a) is the first three charge and discharge data at 100mA / g charge and discharge current dens...
Embodiment 3
[0035] 100mmol of Ni(NO 3 ) 2 ·6H 2 O and 100mmol of Co(NO 3 ) 2 ·6H 2 O mix evenly, add 80mL of distilled water, fully stir for 1 hour, then add 50mL of methanol and 3g of polyvinylpyrrolidone, fully stir for 1 hour, then add 25g of urea, mix well, pour into a 100mL reaction kettle, and keep at 160°C for 18 Hours; after the reaction, cool down to room temperature naturally, wash with a large amount of distilled water, dry into solid powder in a blast drying oven at 80°C, put the powder into a muffle furnace, and heat up to 350°C at a speed of 5°C / min. ℃, and then keep it warm for 2 hours to obtain the nickel-cobalt oxide electrode material.
[0036] The electrode material topography figure that embodiment 3 prepares is as Figure 4 (a), Figure 4 (b) and Figure 4 (c) shown. The cycle performance test after making the battery is as follows: Figure 5 (a) and Figure 5 (b) shown. According to the test results, under the current density of 100mA / g, the first dischar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com