Peptide, peptide derivatives, medicinal salts of peptide, medicine composition and application of peptide and peptide derivatives
A technology of peptide derivatives and compositions, which is applied in the field of polypeptides and their derivatives, pharmaceutically acceptable salts and pharmaceutical compositions, can solve the problems of short plasma half-life, achieve long plasma half-life, good stability, and improve pancreatic islet The effect of the function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1 Production of Polypeptides
[0133] This example describes the production of polypeptides
[0134] All peptides used in this study were synthesized using 9-fluorenylmethyl chloroformate (Fmoc) solid-phase synthesis. Briefly, a weighed amount of 2-chlorotrityl chloride resin (1.6 mmol / g) was dissolved in dichloromethane (DCM). For C-terminally amidated peptides of interest, use Rink amide resin instead of 2-chlorotrityl chloride resin. For coupling reactions in the presence of hydroxybenzotriazole (Sigma Chemicals, Inc., St. Louis, MO, USA) in dimethylformamide (DMF), preactivated Fmoc-amino acids were used. The entire synthesis process uses an excess of amino acids. Deprotection of the Fmoc group in 20% piperidine in DMF leads to chain extension reactions. When the chain extension reaction was completed, the Fmoc protecting group was removed from the N-terminus of the polypeptide using DMF containing 25% piperidine, and then washed four times with DMF solu...
Embodiment 2
[0138] Embodiment 2 Stability experiment of peptide
[0139] This example describes stability experiments of peptides under various conditions.
[0140] Accurately weigh a certain amount of the selected peptide, dissolve it in distilled water to a concentration of 5 mg / mL, and use it as a stock solution to examine the stability of the peptide in the culture medium. The stock solution was diluted to 0.25 mg / mL using F-12K medium (GIBCO-BRL, Gaithersburg, Maryland, USA) as a working solution. Transfer each 100 µL of working solution to a separate vial. After the vials were placed in a 37°C incubator for 0, 24, 48 and 72 hours, quantitative analysis was performed using HPLC.
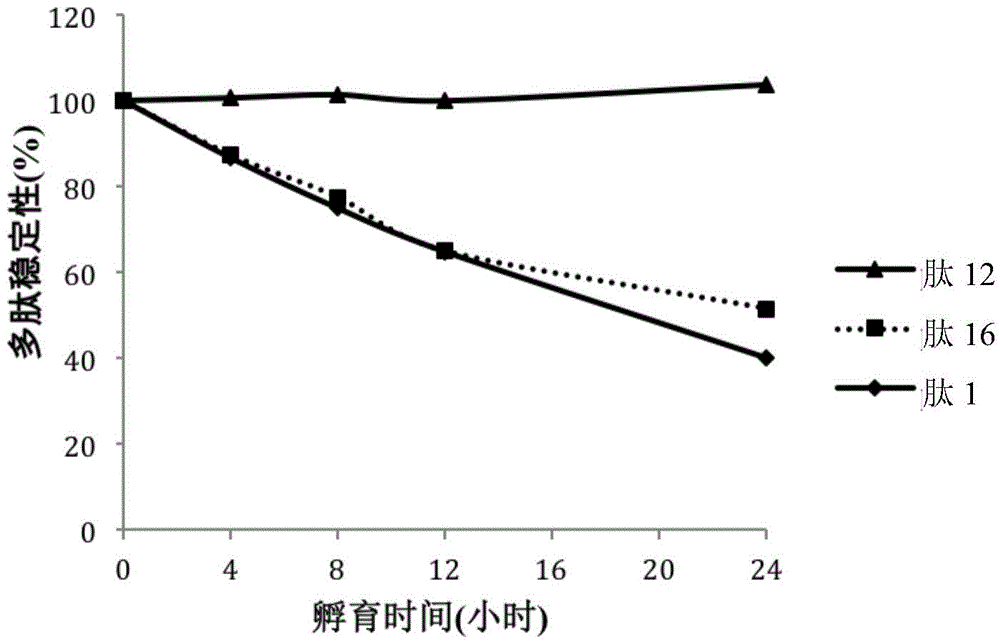
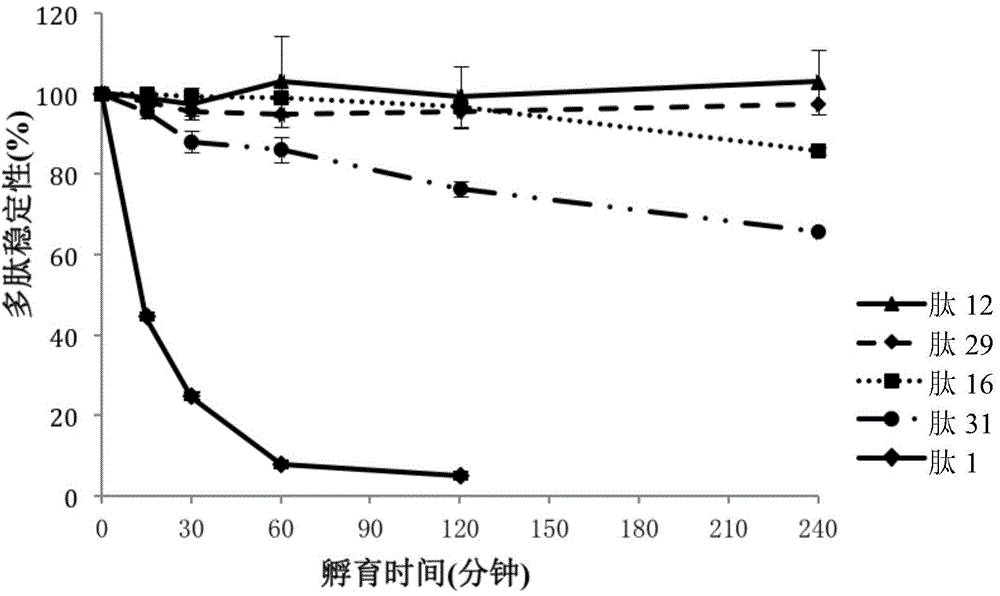
[0141] Table 5 shows the stability of the compounds in the medium. Table 5 shows the stability comparison in culture medium of INGAP-PP (peptide 1) and selected polypeptides, peptide 12 and peptide 16 (see Table 2). in particular, figure 1 Stability comparisons of the INGAP peptide (peptide 1) and sele...
Embodiment 3
[0168] Example 3 Effect of Peptides on Glucose-Stimulated Insulin Secretion
[0169] This example describes the effect of peptides on glucose-stimulated insulin secretion (GSIS).
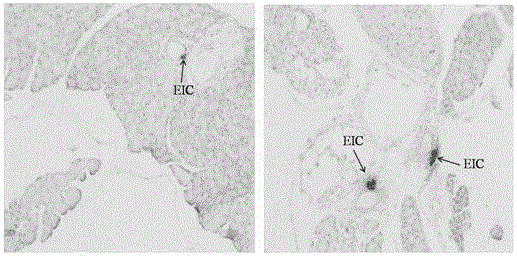
[0170] Pancreatic tissue was extracted from male adult Sprague-Dawley (SD) rats. After 7 days of acclimatization, the animals were sacrificed by cervical dislocation, and the whole pancreas was harvested, and the islets were digested with collagenase. After digestion, islets were stored at 37°C in a humidified environment at pH 7.4 containing 10% (vol / vol) fetal bovine serum, 1% penicillin / streptomycin, 10 mM glucose (5% CO 2 / 95%O 2) in the medium of RPMI 1640 (Carlsbad, California, USA) and divided into the following groups: without adding any compound (control group), adding 100 nM glucagon-like peptide-1 (GLP-1 group) , adding 10 μg / mL peptide 1 group, peptide 12 group, peptide 16 group, see Table 10 below.
[0171] Table 10
[0172]
[0173]
[0174] in CO 2 / O 2 (5 / 95%), 37 ℃ envi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com