A kind of jellyfish jellyfish serine protease inhibitor and its coding gene and application
A technology of serine protease and jellyfish jellyfish, which is applied in the field of biomedicine, can solve the problems that have not been reported on jellyfish Kazal-type serine protease inhibitors, etc., and achieve significant serine protease activity, inhibition of antibacterial activity, and inhibition of serine proteases active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
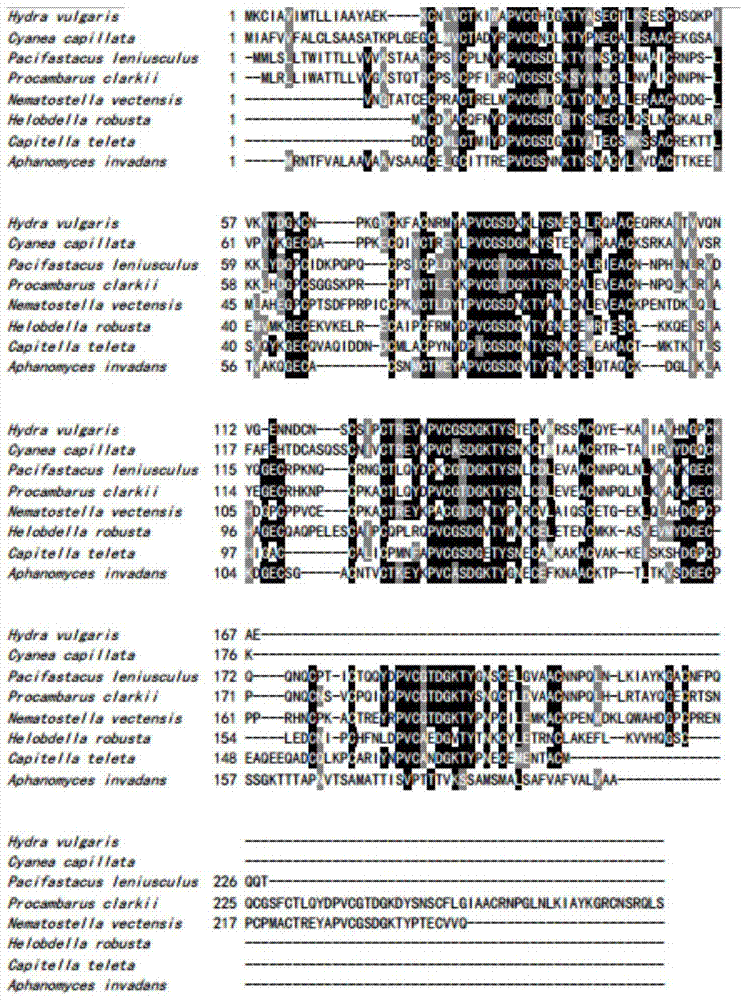
[0058] Example 1: Screening and sequence analysis of jellyfish serine protease inhibitors
[0059] 1) The extraction of total RNA from the tentacles of A. phagina jellyfish was carried out according to the instructions of the Trizol kit from Invitrogen, and the protein was removed with chloroform to obtain about 7 μg of total RNA;
[0060] 2) The isolation of mRNA was carried out according to the instructions of Oligotex mRNA Spin-column Kit of QIANGEN Company, and the synthesis of cDNA was carried out according to the instructions of SMART cDNA Library Construction Kit of Clontech Company;
[0061]3) Insert the cDNA into the pUC19 plasmid vector (purchased from Takara Company), and then transform it into Escherichia coli DH5α (purchased from Beijing Bomaide Company), spread it on a 15cm petri dish for blue-white screening, and then form the tentacles of jellyfish jellyfish Tissue cDNA library.
[0062] The library has a total of 1923 colonies, including 35 coeruleus, a recom...
Embodiment 2
[0064] Example 2: Construction of a recombinant expression plasmid for Aequosia habilis serine protease inhibitors and recombination of engineering bacteria
[0065] 1) A pair of primers were designed and synthesized according to the sequence of the serine protease inhibitor gene sequence of A. jellia habilis and the restriction site of the prokaryotic expression vector pGEX-6P-1 (purchased from Novagen), wherein the upstream primer contains the restriction site EcoR I (GAATTC), the downstream primer contains restriction site XhoI (CTCGAG), the sequences of the two primers are as follows:
[0066] Upstream primer: 5'CGGAATTCATGACCAAGCCATT 3' (SEQ ID NO:3)
[0067] Downstream primer: 5'CCGCTCGAGTTTTCTGCATTG 3'(SEQ ID NO:4)
[0068] After the gradient PCR experiment, 55.5°C was selected as the optimal annealing temperature, and the target gene was amplified by PCR. The PCR reaction conditions were: 95°C for 5 min; 95°C for 30 sec, 55.5°C for 30 sec, 68°C for 1 min, 38 cycles; ...
Embodiment 3
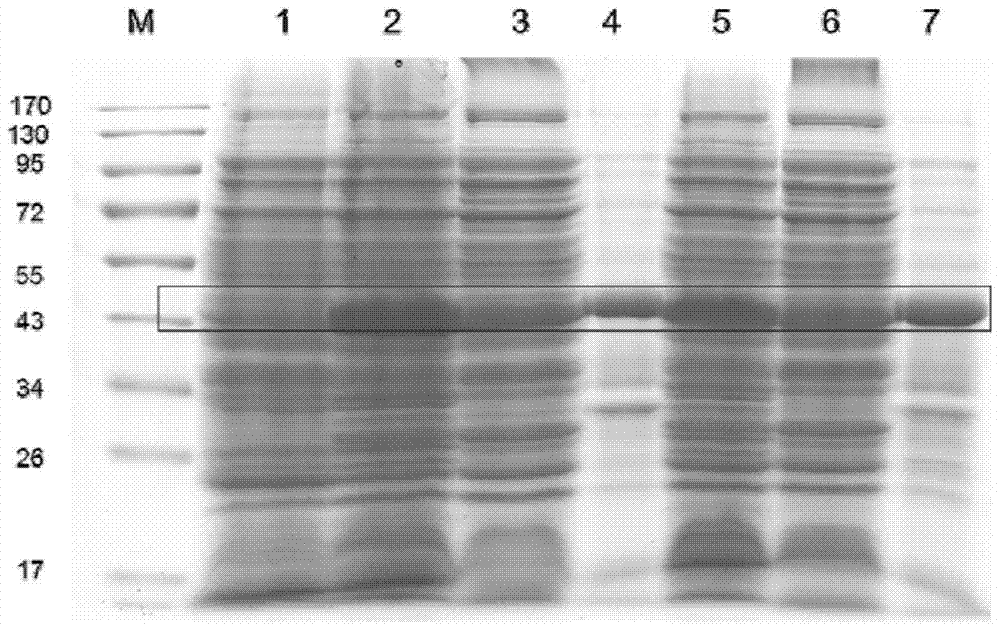
[0071] Example 3: Expression of Serine Protease Inhibitors from Aequorea habilis
[0072] Add the recombinant Escherichia coli Rosetta (DE3).pLysS bacterial liquid with correct sequencing to the liquid LB medium containing ampicillin (100 μg / ml) and chloramphenicol (34 μg / ml), and cultivate it to OD at 37 ° C and 250 rpm on a shaker 600 When it was 0.6-0.8, the inducer IPTG was added for induction.
[0073] The optimal expression condition of the recombinant protein was determined as follows: induction at 12° C., 1 mM IPTG, and 150 rpm for 7 hours. After induction, the bacteria were collected by centrifugation, and the recombinant protein could be expressed in a soluble form under the induction conditions by SDS-PAGE electrophoresis, and the molecular weight was consistent with the predicted value (about 45kDa after adding the GST tag), as shown in image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com