Genetic engineering bacterial strain for expressing bacillus subtilis laccase in strain cell and method for realizing secreting and expressing laccase in bacterial strain
A technology of Bacillus subtilis and genetically engineered strains, applied in the directions of microorganism-based methods, biochemical equipment and methods, enzymes, etc., can solve the problems of secondary environmental pollution, low degradability, high cost, and achieve good reference value, The effect is remarkable and the method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0027] Specific embodiment one: the method for constructing the genetically engineered bacterial strain expressing Bacillus subtilis laccase intracellularly in the present embodiment is carried out according to the following steps:
[0028] 1. Extraction of Bacillus genomic DNA and cloning of bacterial laccase gene:
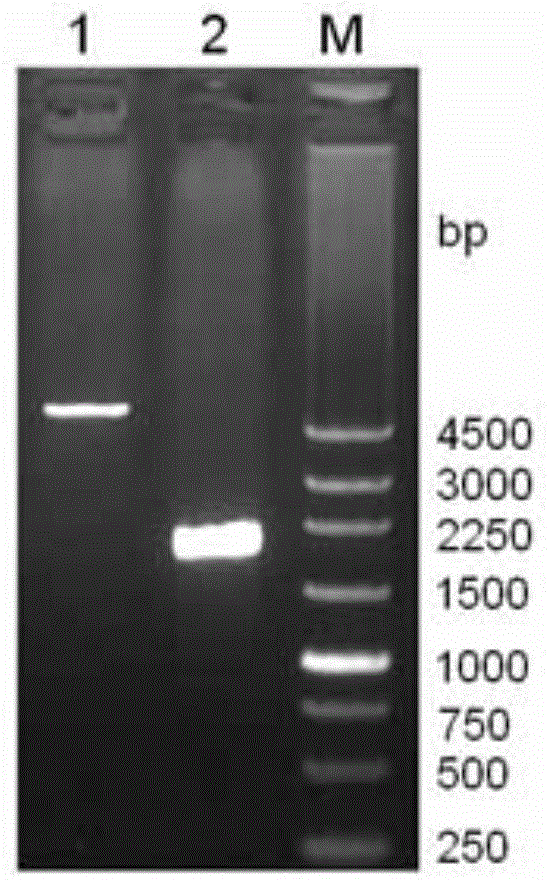
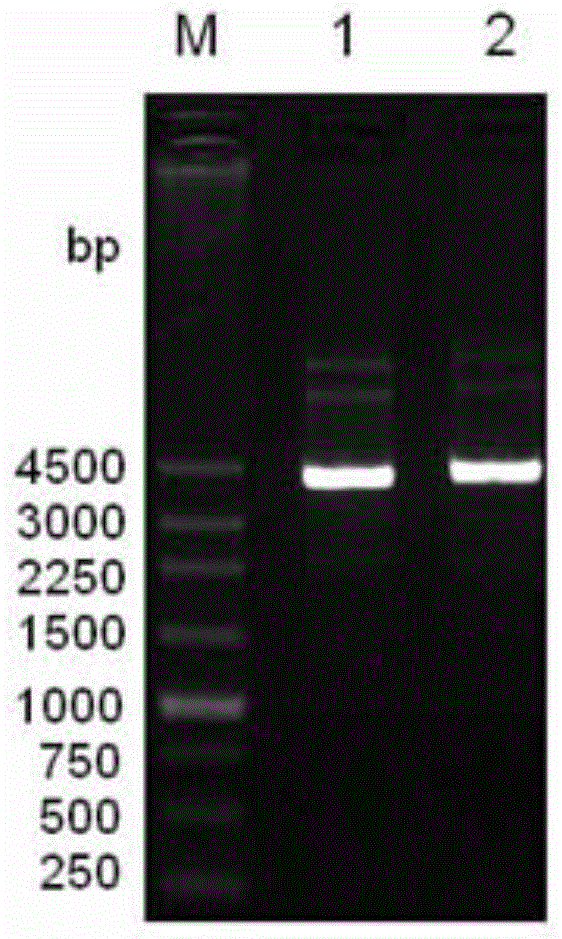
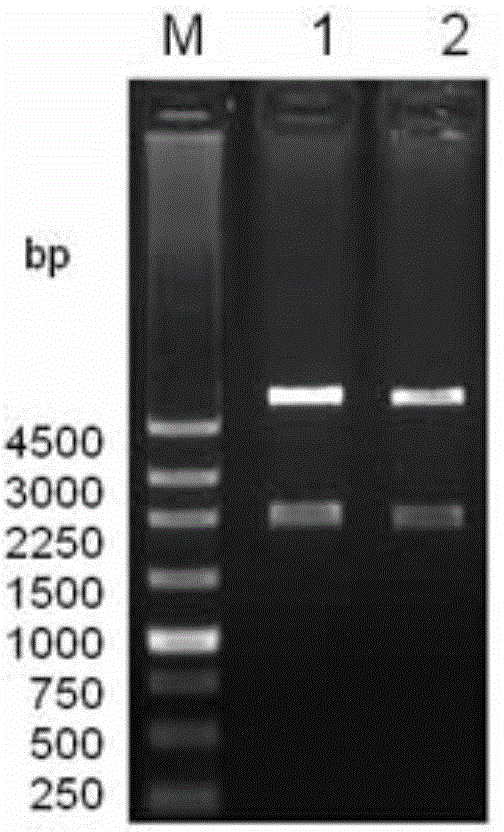
[0029] The genomic DNA of Bacillus subtilis LS02 was extracted, using the genomic DNA as a template, using primers LS02-F and LS02-R for PCR amplification, introducing restriction sites NdeI and BamhI at both ends of the laccase gene sequence, and the amplified product was purified Afterwards, it was connected to the pEASY-Blunt cloning vector to obtain the recombinant vector pEASY / CotA. Using pEASY / CotA as a template, the point mutation primers LS02-Mut-F and LS02-Mut-R were used to amplify the whole plasmid by PCR, and the CotA DNA sequence was The 927th base T was mutated into base C. On the basis of not changing the amino acid sequence of the CotA protein, th...
specific Embodiment approach 2
[0046] Specific embodiment two: this embodiment realizes the method for inducing the secretion and expression of laccase by the bacterial strain described in specific embodiment one, and proceeds according to the following steps:
[0047] 1. Insert the recombinant expression strain PSD glycerol into LB medium containing 50mg / L Amp, and culture overnight at 37°C and 180rpm for 12-14h to obtain the bacterial solution;
[0048] 2. Inoculate the bacterial liquid obtained in step 1 into fresh LB medium containing 50mg / L Amp according to 1% inoculum amount, and cultivate to OD at 30°C and 180rpm 600 is 0.8, add 0.1mM IPTG and 0.1mM CuSO 4 , cultured at 120 rpm at 25°C for 4 hours, and then cultured statically at 30°C; from the time of adding the IPTG inducer, samples were taken every 4 hours to detect the laccase activity of the medium supernatant, intracellular, and periplasmic space, and simultaneously prepared Protein samples were used for SDS-PAGE analysis.
[0049] Laccase ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com