Fluorescent probes based on pyrene, and preparation method and application thereof
A fluorescent probe and azido-based technology, applied in the field of chemistry, can solve the problems of few reports and unreported fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) One-step reaction synthesizes two kinds of reaction formulas based on pyrene-based fluorescent probes:
[0043]
[0044] (2) The specific steps of one-step reaction synthesis two kinds of fluorescent probes based on pyrene:
[0045] Weigh 100mg of 1,8-diethynylpyrene and 273mg of 2-azido-N-(quinolin-8-yl)acetamide, dissolve in 10ml THF:H 2 O=4:1, stirred overnight at 60°C. Ammonia was added for extraction, the solvent was distilled off under reduced pressure, and the obtained solid was purified by silica gel chromatography, using pure dichloromethane as the eluent to obtain 73 mg of light yellow solid compound 1 with a yield of 38%. Methane=1:15 (volume ratio) was used as the eluent to obtain 43 mg of compound 2 as a yellow solid with a yield of 15%.
Embodiment 2
[0047] Weigh 200mg of 1,8-diethynylpyrene and 545mg of 2-azido-N-(quinolin-8-yl)acetamide, dissolve in 10ml THF:H 2O=4:1, stirred overnight at 65°C. Ammonia was added for extraction, the solvent was distilled off under reduced pressure, and the obtained solid was purified by silica gel chromatography, using pure dichloromethane as the eluent to obtain 156 mg of light yellow solid compound 1 with a yield of 41%. Methane=1:16 (volume ratio) was used as the eluent to obtain 101 mg of compound 2 as a yellow solid, with a yield of 18%.
Embodiment 3
[0049] Weigh 250mg of 1,8-diethynylpyrene and 680mg of 2-azido-N-(quinolin-8-yl)acetamide, dissolve in 10ml THF:H 2 O=4:1, stirred overnight at 66°C. Ammonia was added for extraction, the solvent was distilled off under reduced pressure, and the obtained solid was purified by silica gel chromatography, using pure dichloromethane as the eluent to obtain 215 mg of light yellow solid compound 1 with a yield of 45%. Methane=1:13 (volume ratio) was used as the eluent to obtain 155 mg of compound 2 as a yellow solid, with a yield of 22%.
[0050] Basic data of the fluorescent probes 1 and 2 of Embodiment 1 to Embodiment 3:
[0051] Fluorescent probe 1:
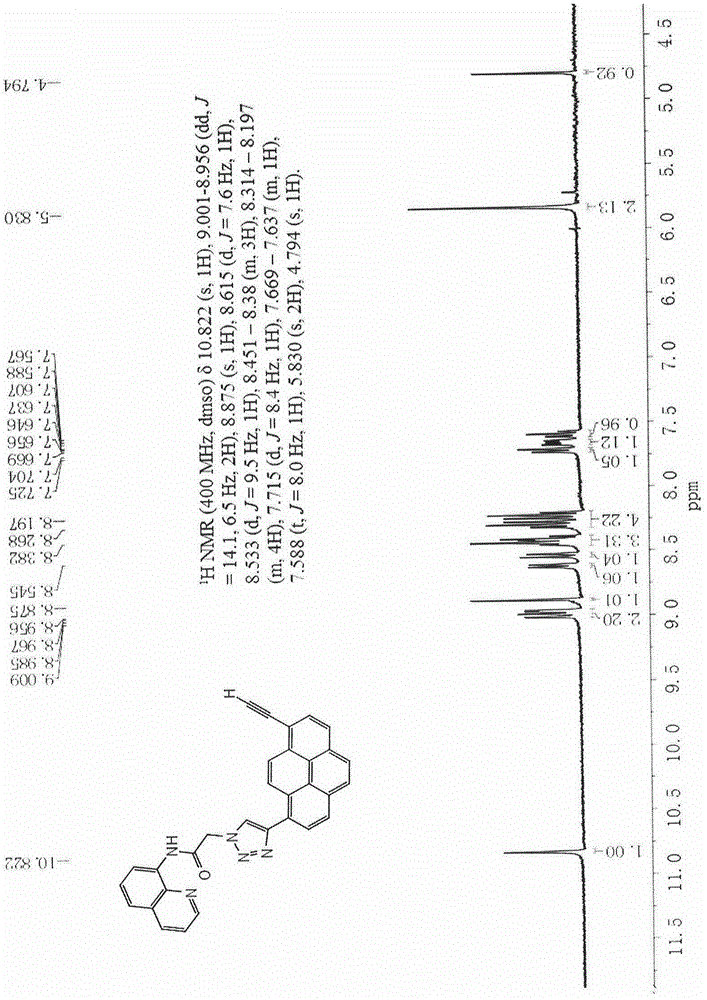
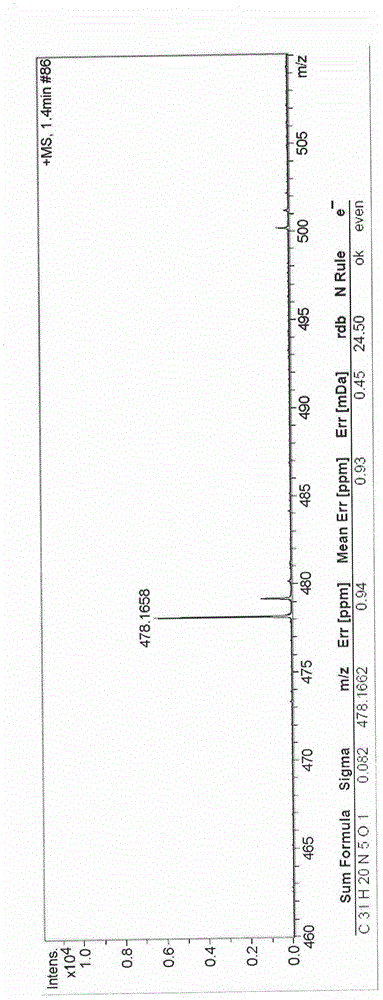
[0052] 1 H NMR (400MHz, DMSO-d 6 )δ10.822(s, 1H), 9.001-8.956(dd, J=14.1, 6.5Hz, 2H), 8.875(s, 1H), 8.615(d, J=7.6Hz, 1H), 8.533(d, J =9.5Hz, 1H), 8.451-8.38(m, 3H), 8.314-8.197(m, 4H), 7.715(d, J=8.4Hz, 1H), 7.669-7.637(m, 1H), 7.588(t, J=8.0Hz, 1H), 5.830(s, 2H), 4.794(s, 1H). (such as figure 1 )
[0053] 13 C NMR (101MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com