Polypeptide, derivative of polypeptide, pharmaceutical salt of polypeptide, pharmaceutical composition and application of polypeptide or derivative of polypeptide
A technology of peptide derivatives and compositions, applied in the field of polypeptides and derivatives of the polypeptides, can solve the problems of short plasma half-life and the like, and achieve the effects of long plasma half-life, good stability, and improvement of islet function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] The production of embodiment 1 polypeptide
[0143] This example describes the production of polypeptides
[0144] All peptides used in this study were synthesized using 9-fluorenylmethyl chloroformate (Fmoc) solid-phase synthesis. Briefly, a weighed amount of 2-chlorotrityl chloride resin (1.6 mmol / g) was dissolved in dichloromethane (DCM). For C-terminally amidated peptides of interest, use Rink amide resin instead of 2-chlorotrityl chloride resin. For coupling reactions in the presence of hydroxybenzotriazole (Sigma Chemicals, Inc., St. Louis, MO, USA) in dimethylformamide (DMF), preactivated Fmoc-amino acids were used. The entire synthesis process uses an excess of amino acids. Deprotection of the Fmoc group in 20% piperidine in DMF leads to chain extension reactions. When the chain extension reaction was completed, the Fmoc protecting group was removed from the N-terminus of the polypeptide using DMF containing 25% piperidine, and then washed four times with DM...
Embodiment 2
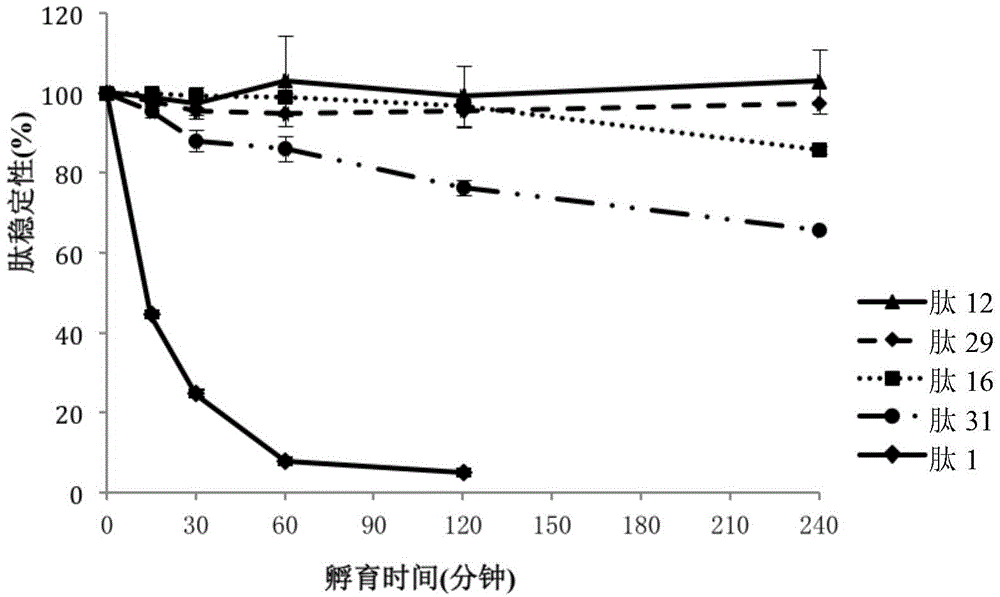
[0148] Stability experiment of embodiment 2 peptide
[0149] This example describes stability experiments of peptides under various conditions.
[0150] Accurately weigh a certain amount of the selected peptide, dissolve it in distilled water to a concentration of 5 mg / mL, and use it as a stock solution to examine the stability of the peptide in the culture medium. The stock solution was diluted to 0.25 mg / mL using F-12K medium (GIBCO-BRL, Gaithersburg, Maryland, USA) as a working solution. Transfer each 100 µL of working solution to a separate vial. After the vials were placed in a 37°C incubator for 0, 24, 48 and 72 hours, quantitative analysis was performed using HPLC.
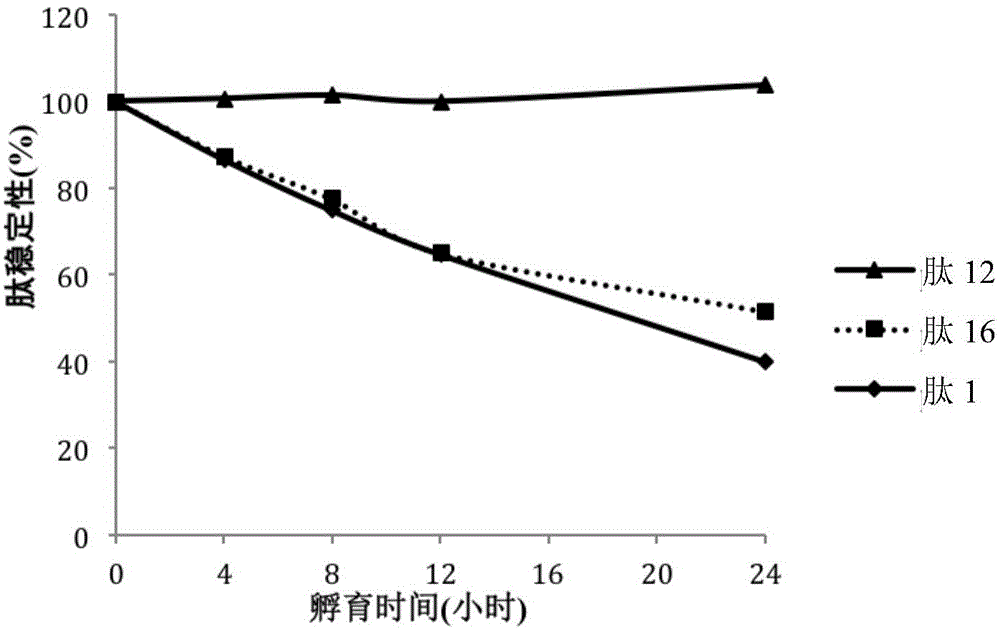
[0151] Table 5 shows the stability of the compounds in the medium. Table 5 shows the stability comparison in culture medium of INGAP-PP (peptide 1) and selected polypeptides, peptide 12 and peptide 16 (see Table 2). in particular, figure 1 Stability comparisons of the INGAP peptide (peptide 1) and sele...
Embodiment 3
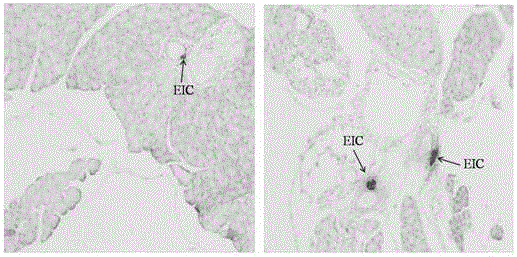
[0183] Example 3 Effects of Peptides on Glucose-Stimulated Insulin Secretion
[0184] This example describes the effect of peptides on glucose-stimulated insulin secretion (GSIS).
[0185] Pancreatic tissue was extracted from male adult Sprague-Dawley (SD) rats. After 7 days of acclimatization, the animals were sacrificed by cervical dislocation, and the whole pancreas was harvested, and the islets were digested with collagenase. After digestion, islets were stored at 37°C in a humidified environment at pH 7.4 containing 10% (v / v) fetal bovine serum, 1% penicillin / streptomycin, 10 mM glucose (5% CO 2 / 95%O 2 ) in the medium of RPMI 1640 (Carlsbad, California, USA) and divided into the following groups: without adding any compound (control group), adding 100 nM glucagon-like peptide-1 (GLP-1 group) , adding 10 μg / mL peptide 1 group, peptide 12 group, peptide 16 group, see Table 11 below.
[0186] Table 11
[0187]
[0188]
[0189] in CO 2 / O 2 (5 / 95%), 37 ℃ enviro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com