Myocardial infarction rapid detection kit and preparation method thereof
A detection kit and kit technology, applied in the field of rapid detection kits for myocardial infarction and its preparation, can solve the problems of low accuracy, low sensitivity, and low diagnostic accuracy, and achieve rapid response, high sensitivity, and specificity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation method and detection method of embodiment 1 human myeloperoxidase-cardiac fatty acid binding protein-troponin I triple colloidal gold rapid quantitative detection kit (hereinafter referred to as MPO-FABP3-cTnI triple colloidal gold kit)
example 1
[0057] Example 1: Preparation of MPO-FABP3-cTnI Triple Colloidal Gold Kit
[0058] 1. Main material
[0059] MPO, FABP3 and cTnI standard products: National Institute of Inspection and Quarantine; MPO, FABP3 antibodies and specific paired antibodies are products of Shanghai Enzyme Biotechnology Co., Ltd., cTnI antibodies and specific paired antibodies are products of Shanghai Lingchao Biotechnology Co., Ltd.; Avidin: Thermo product; goat anti-mouse IgG antibody: product of Hangzhou Qitai Biotechnology Co., Ltd.; chloroauric acid: product of Sigma Company; nitrocellulose (NC) membrane: SARTORIUS (Germany), CN140; bovine serum albumin ( BSA), polyethylene glycol PEG20000, hydrolyzed casein: Sigma products. Other commonly used reagents are analytical reagents.
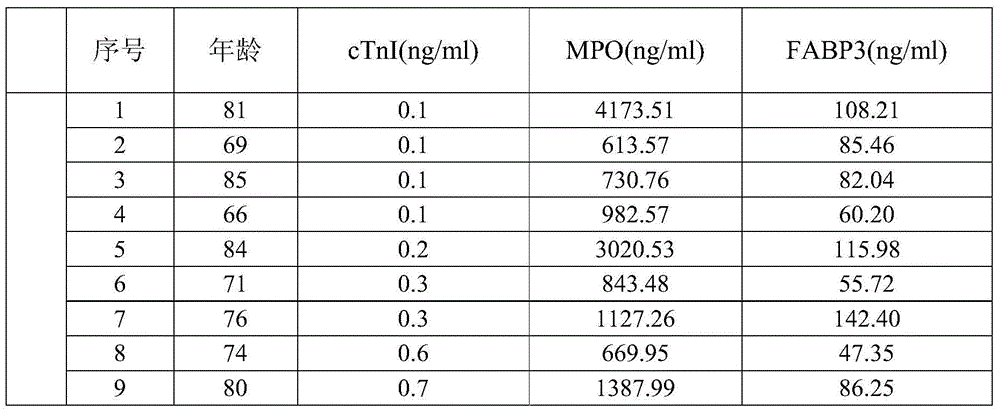
[0060] Clinical samples: Obtained by the company in relevant hospitals, a total of 200 samples, MPO, FABP3 and cTnI content distribution intervals were 6.25-400ng / ml, 3.125-200ng / ml, 0.1-25ng / ml between the fixed value ...
example 2
[0070] Example 2: Preparation of MPO-FABP3-cTnI Triple Colloidal Gold Kit
[0071] 1. Main material
[0072] MPO, FABP3 and cTnI standard products: National Institute of Inspection and Quarantine; MPO, FABP3 antibodies and specific paired antibodies are products of Shanghai Enzyme Biotechnology Co., Ltd., cTnI antibodies and specific paired antibodies are products of Shanghai Lingchao Biotechnology Co., Ltd.; Mouse IgG antibody: product of Hangzhou Qitai Biotechnology Co., Ltd.; chloroauric acid: product of Sigma; nitrocellulose (NC) membrane: SARTORIUS (Germany), CN140; product of Sartorius, Germany; bovine serum albumin (BSA) , polyethylene glycol PEG20000, hydrolyzed casein: a product of Sigma. Other commonly used reagents are analytical reagents.
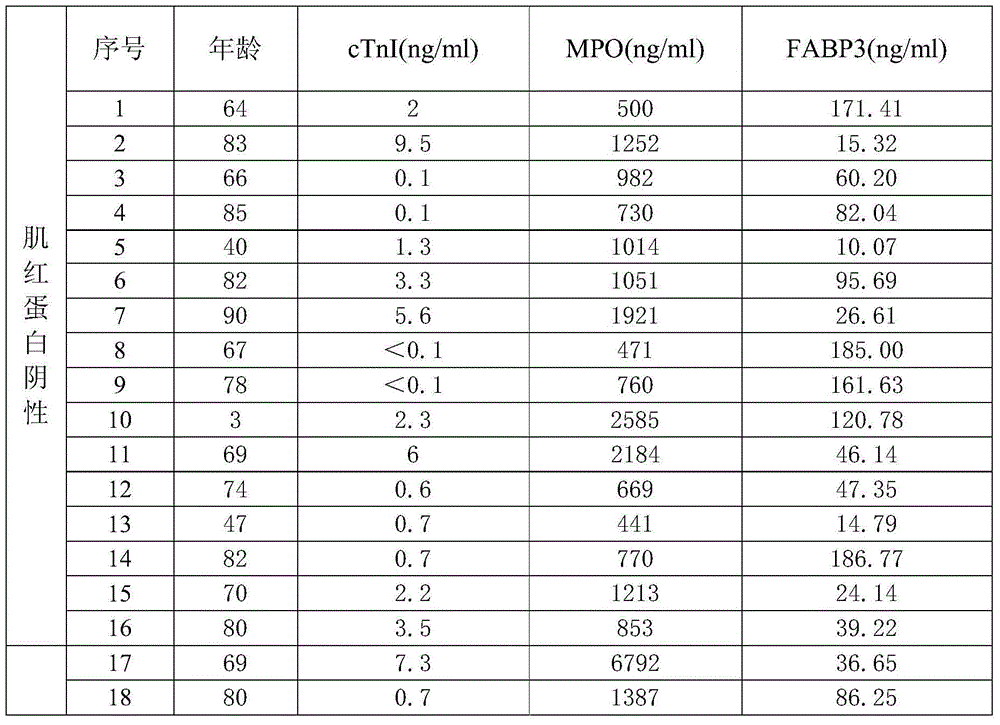
[0073] Clinical samples: Obtained by the company in relevant hospitals, a total of 200 samples, MPO, FABP3 and cTnI content distribution intervals were 6.25-400ng / ml, 3.125-200ng / ml, 0.1-25ng / ml between the fixed value serum. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com