Triphenylamine-BODIPY derivative organic dye and preparation method thereof
A technology for dipyrromethene and organic dyes, applied in the direction of organic dyes, methine/polymethine dyes, chemical instruments and methods, etc., can solve the problems of pyrrole ring self-polymerization, catalytic decomposition, and low synthesis yield, and achieve reaction The conditions are easy to control, the overall yield is high, and the reaction conditions are more effective.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of Dye III
[0046] (1) Add 4-bromotriphenylamine (650mg, 2mmol) and 5ml dimethylformamide (DMF) into a 100ml three-necked flask, stir it with a magnetic force, put it in an ice-water bath to 0°C, and slowly add POCl dropwise into the three-necked flask 3 (9ml, 20mmol), and a large amount of solids were formed, after the dropwise addition, the temperature was slowly raised to 60°C, and the temperature was controlled to continue the reaction for 2h, then the reaction was stopped, cooled to room temperature, poured into water, CH 2 Cl 2 Extract, wash with saturated NaCl solution 20ml×3 and distilled water 20ml×3 in sequence, collect the organic phase, dry over anhydrous magnesium sulfate, spin off the solvent under reduced pressure, and the crude product is subjected to column chromatography with petroleum ether as the eluent to obtain the intermediate 620mg of 2(4-bromo-4',4"-diformyltriphenylamine) light yellow crystals, yield 81%. 1 HNMR (400MHz, CDCl 3 TM...
Embodiment 2
[0050] Synthesis of Dye II
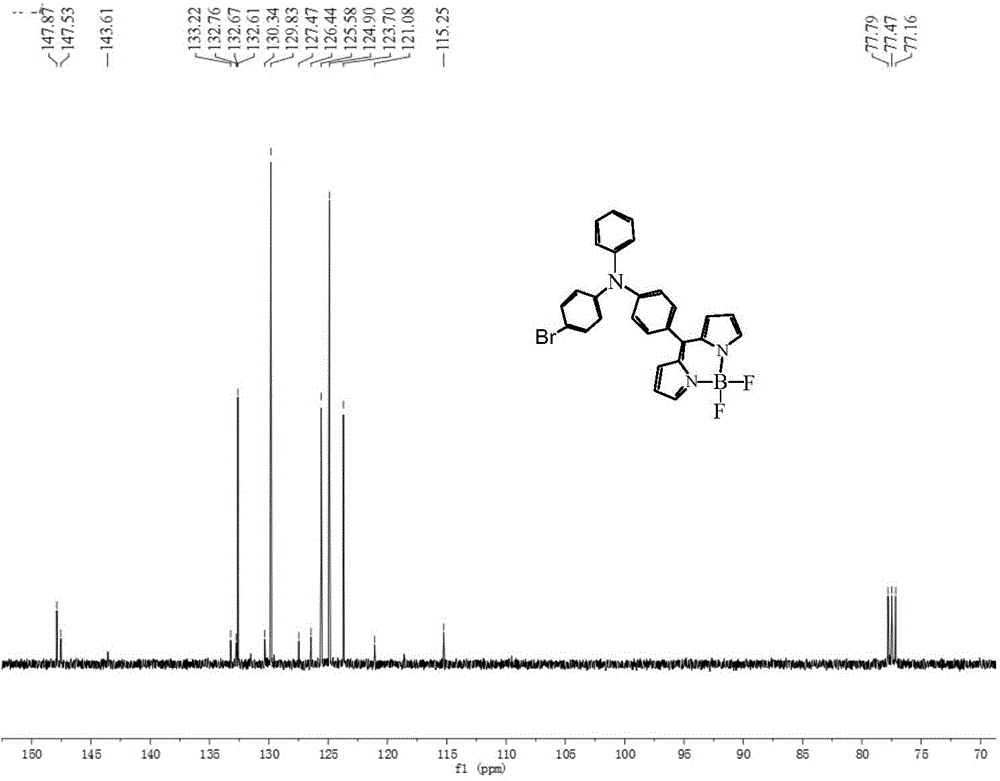
[0051] The synthetic method of dye II is similar to that of dye III, except that in the step (1) reaction of synthetic dye III, POCl 3 The molar ratio with DMF is 1:1, and other reaction conditions are the same, and the intermediate 1 is 4-bromo-4'-formyltriphenylamine. The yield of dye II was 81%, and the melting point was 208-210°C. 1 HNMR (400MHz, CDCl 3 TMS,ppm):δ:7.91(s,2H),6.93-7.47(m,15H),6.56(s,2H); 13 CNMR (100MHz, CDCl 3 , TMS, ppm): δ: 147.87, 147.53, 143.61, 133.22, 132.76, 132.67, 132.61, 130.34, 129.83, 127.47, 126.44, 125.58, 124.90, 123.70, 121.08, 115.25. 494.159(obs.)[M-F]+,calcd.avg.mass:513.081.
Embodiment 3
[0053] Synthesis of Dye IV
[0054] Add dye II (200mg, 0.38mmol), 2-octyl-5-(tributyltin)thiophene (200mg, 0.40mmol), 20ml DMF, 120mg of tetrakis(triphenylphosphine)palladium, and extract Vacuum, N 2 Gas protection, temperature control at 90 ° C for 48 hours. Stop the reaction, cool, pour into water, CH 2 Cl 2 Extraction, organic phase anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure. The crude product was separated and purified by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate = 15:1, and dye IV was obtained as a purple-black solid viscous substance, 182mg, yield 76%, melting point 93-95°C. 1 HNMR (400MHz, CDCl 3 ,TMS,ppm):δ:6.73-7.67(m,19H),6.39(s,2H),2.79-2.83(t,J=6.5Hz,2H),1.25-1.68(m,12H),0.88(t , J=7.2Hz, 3H). MALDI-TOF-MS: m / z=582.460 (obs.) [M-47]+, calcd.avg.mass: 629.282.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com