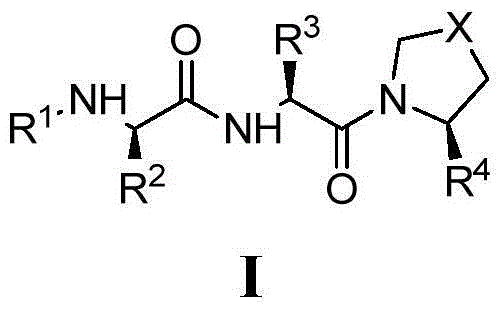
Anti-apoptosis protein inhibitor and application thereof
A technology of apoptosis inhibitory protein and medicinal salt, which is applied in the field of medicine and chemical industry, and can solve the problems of compound efficacy, stability or toxicity limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1 Synthesis
[0128] Dissolve intermediate A-10 (0.5mmol) in as little ethyl acetate as possible, and add 3mol·L -1 Hydrogen chloride / ethyl acetate solution 3mL, react at low temperature for 0.5h, continue stirring at room temperature for 3h. A white solid was precipitated, filtered, washed with ether, and dried to obtain 221 mg of a white solid, namely Monovalent Example 1 (hydrochloride salt form), with a yield of 83.2%. 1 H-NMR (400MHz, DMSO-d 6 ), δ(ppm):0.85-0.93(m,5H),1.32-1.34(d,3H,J=7.2Hz),1.36-1.52(m,7H),2.00-2.10(m,2H),2.29- 2.34(m,2H),2.46-2.49(m,3H), 3.80-3.95(m,3H),4.20(s,3H),4.46-4.48(t,1H,J=8.0Hz),5.23-5.26( m,1H),7.45-7.48(m,3H),8.32-8.35(m,2H),8.66-8.69(d,1H,J=8.0Hz),8.86(br s,1H),9.36(br s, 1H),12.89(s,1H),MS m / e:520.3([M+1] + ).
Embodiment 23
[0129] Example 23 Synthesis
[0130] Add intermediate A-10 (80mg, 0.138mmol), 1,6-dibromohexane (100mg, 0.415mmol) and catalytic amount of potassium iodide to the 10mL closed reaction tube dedicated to the fully automatic combined microwave synthesis instrument (product of CEM company) and 3 mL of acetone, and set the reaction conditions at 120° C. / 20 minutes. The reaction system was evaporated to dryness under reduced pressure, and then separated by column chromatography. The elution solvent was dichloromethane / methanol (20:1), and 62 mg of oil was obtained with a yield of 72.5%.
[0131] Dissolve this oil in as little ethyl acetate as possible, and add 3mol·L -1 Hydrogen chloride / ethyl acetate solution 3mL, react at low temperature for 0.5h, continue stirring at room temperature for 1h. A white solid precipitated, was filtered, and the solid was washed with ether. After drying, 46 mg of white solid was obtained, that is, the divalent compound Example 23 (dihydrochlor...
Embodiment 7
[0141] Example 7 Synthesis
[0142] Dissolve intermediate B-7 (1mmol, 536mg) in as little ethyl acetate as possible, and add 3mol·L -1 Hydrogen chloride / ethyl acetate solution 3mL, react at low temperature for 0.5h, continue stirring at room temperature for 3h. A white solid was precipitated, filtered, washed with ether, and dried to obtain 402 mg of a white solid, that is, Monovalent Example 7 (hydrochloride salt form), with a yield of 80.2%. 1 H-NMR (400MHz, DMSO-d 6 ), δ(ppm):0.80-0.91(d,6H,J=6.6Hz),1.33-1.35(d,3H,J=6.8Hz),2.15-2.21(m,2H),2.45(s,3H) ,2.50-2.57(m,1H),3.60(brs,1H),3.72(brs,1H),3.83-3.91(m,3H),4.47-4.50(m,2H),5.35-5.37(m,1H ),7.56-8.73(m,8H),8.94(brs,1H),9.58(br s,1H); MS m / e:464.4([M+1] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com