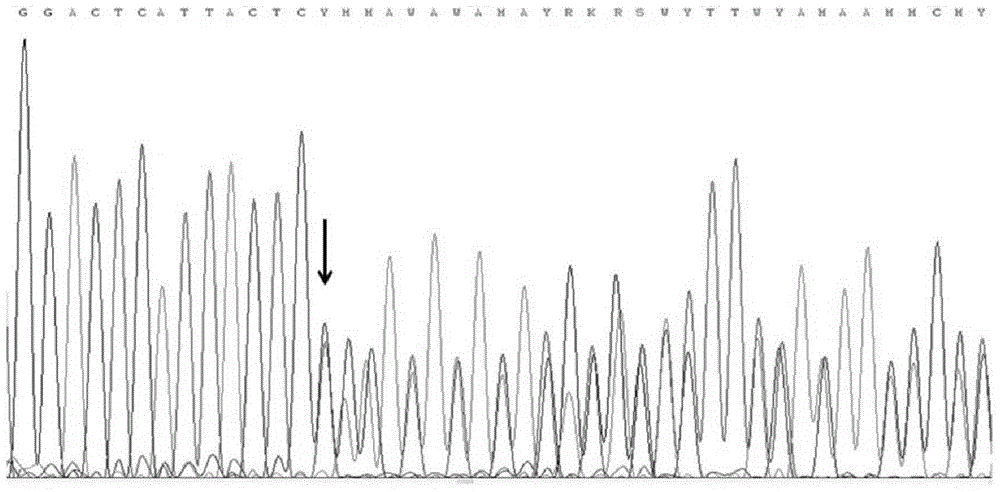
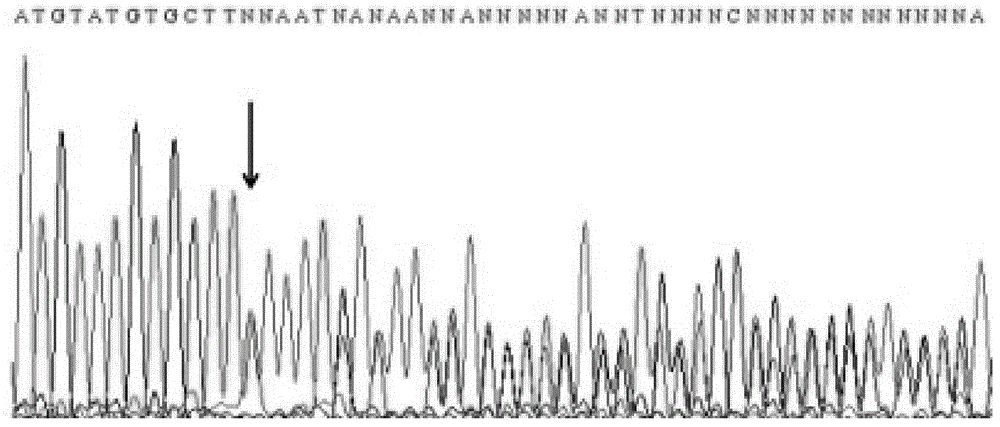
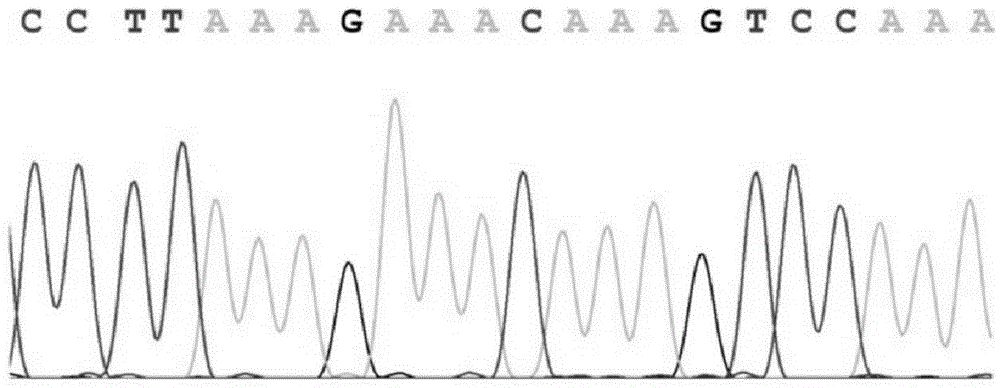
Method and primer for detecting mutation sites of all exon sequences of human BRCA1 and BRCA2 genes
A mutation site and whole exon technology, which is applied in the field of detection of mutation sites in the whole exon sequence of human BRCA1 and BRCA2 genes, can solve the problems of inability to sequence the coding sequences of BRCA1 and BRCA2 genes, so as to reduce the risk of disease and improve the sensitivity The effect of high, high specificity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Primer sequences for detection of exon mutations in BRCA1 and BRCA2 genes
[0042] (1) Primers for detection of BRCA1 gene exon mutation sites
[0043] Including the sequence shown in SEQ ID NO: 001-062, it is the forward and reverse primers for amplifying and covering the exon mutation site of BRCA1 gene.
[0044] In the detection, first use the above-mentioned forward and reverse primers to amplify the DNA fragment covering the exon mutation site of BRCA1 gene to obtain the amplified products, and then use the above-mentioned amplification primers to sequence the amplified products respectively to obtain the amplified The gene sequence of the augmented product.
[0045] (2) Primers for detection of BRCA2 gene exon mutation sites
[0046] Including the sequence shown in SEQ ID NO: 063-142, it is the forward and reverse primers for amplifying and covering the exon mutation site of BRCA2 gene.
[0047] In the detection, first use the above-mentioned forward and revers...
Embodiment 2
[0049] Kit for detecting mutation sites in exons of BRCA1 and BRCA2 genes
[0050] Including: tissue DNA extraction kit (for example, using Capgemini’s extraction kit); absolute ethanol; detection system PCR reaction solution, sequencing system reaction solution, positive control substance, negative control substance and blank control substance. The detection system PCR reaction solution includes: 2x PCR Buffer; 2mM dNTPs; Roche DNA Polymerase (1U / μl); forward and reverse primers covering the whole exons of BRCA1 and BRCA2 genes shown in SEQ ID NO: 001-142.
[0051] The sequencing system includes: sequencing purification solution, EDTA (1.25mmol), absolute ethanol, 75% ethanol, HIDI (highly deionized formamide), covering all exons of BRCA1 and BRCA2 genes shown in SEQ ID NO: 001-142 Forward and reverse amplification primers of 3.2 μm each, and Bigdye Terminator V3.1 (purchased from Applied Biosystems, USA), wherein the sequencing purification solution includes shrimp alkaline ...
Embodiment 3
[0053] Method for detecting BRCA1 gene and BRCA2 gene mutation sites
[0054] (1) Genomic DNA extraction from blood:
[0055] 1) Draw 500 μL of blood and add 1000 μl of red blood cell lysate, invert and mix well, and place at room temperature for 5 min, during which time, invert and mix several times. Centrifuge at 3000rpm for 5min, suck off the supernatant, leave the white blood cell pellet, add 200μl buffer GA, shake until thoroughly mixed.
[0056] 2) Add 20 μl proteinase K solution and mix well.
[0057] 3) Add 200 μl of buffer solution, mix thoroughly by inversion, place at 70°C for 10 minutes, the solution should become clear, and briefly centrifuge to remove water droplets on the inner wall of the tube cap.
[0058] 4) Add 200 μl of absolute ethanol, vortex fully for 15 seconds, at this time flocculent precipitation may appear, briefly centrifuge to remove water droplets on the inner wall of the tube cap.
[0059] 5) Add the solution and flocculent precipitate obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com