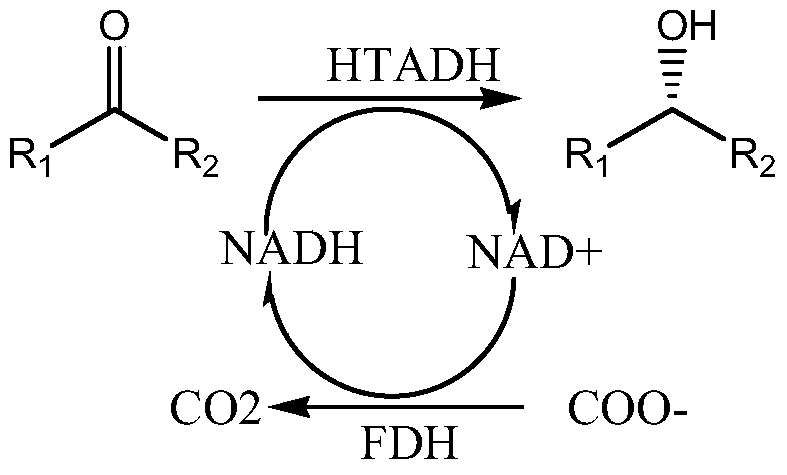
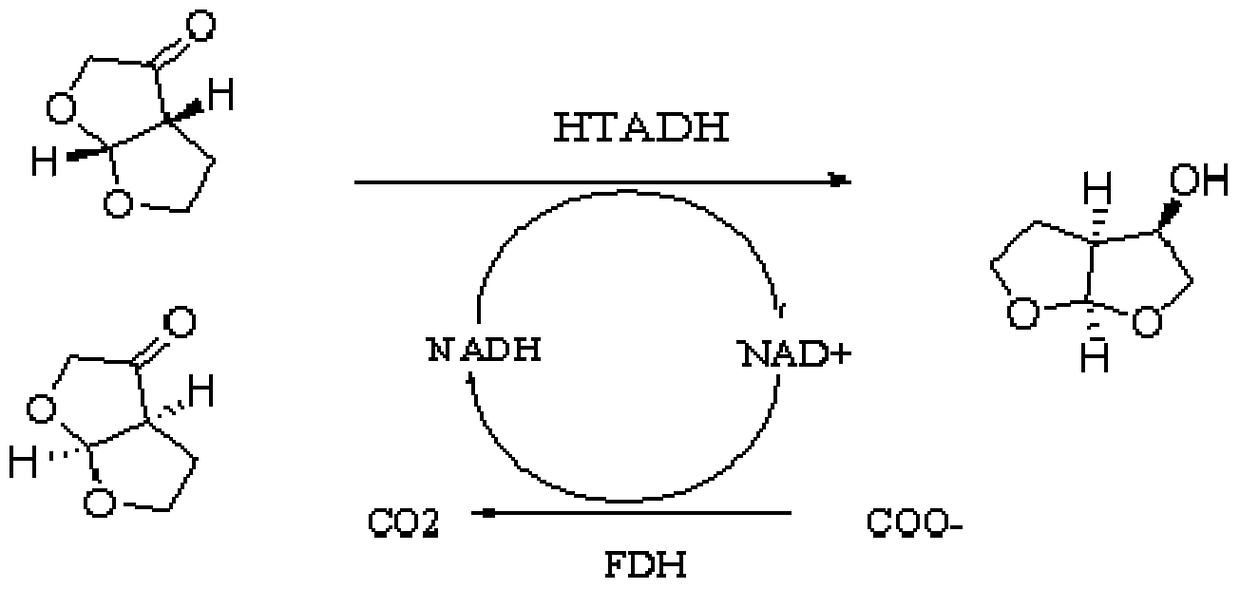
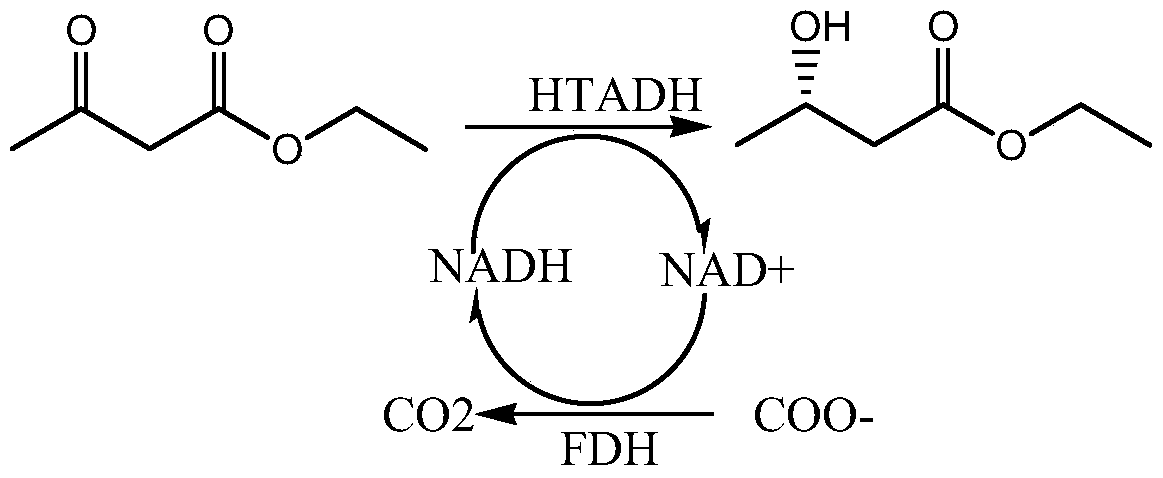
Alcohol dehydrogenase mutant and its application
A technology of alcohol dehydrogenase and mutants, applied in the field of enzyme and enzyme engineering, can solve the problems of high production cost, high diastereoisomer content, cumbersome post-treatment process, etc., and achieve improved enzyme activity and stereoselectivity Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Site-directed saturation mutation of the alcohol dehydrogenase (HTADH) gene (SEQ ID NO: 9) derived from Bacillus stearothermophilus LLD-R strain.
[0058] The amino acid sequence of alcohol dehydrogenase (HTADH) was simulated on the Swiss-model website to simulate the three-dimensional structure of the protein, and then the binding simulation between the substrate and the protein was carried out through Docking. Finally, through Pymol analysis, the selection may be related to the binding of the substrate and NAD. Amino acids associated with NAD proton transport were used as mutant amino acids.
[0059] According to the mutated amino acid and the base sequences on both sides (see the mutation site in Table 1 for the mutated amino acid), use Primmer 5.0 to design the corresponding mutation primers (Table 1). Using the pET22b(+) expression vector containing alcohol dehydrogenase gene (purchased from Novagen, product number 69744) as a template, the complete linear fragment...
Embodiment 2
[0062] Example 2: Cloning and expression of alcohol dehydrogenase mutants
[0063] In order to facilitate the expression and identification of alcohol dehydrogenase mutants, compatible restriction sites were designed at the 5' and 3' ends of the gene. NdeI and XhoI can be used to digest the target gene and pET-22b(+) (other expression plasmids that can express proteins in Escherichia coli can also be used) at the same time, respectively, and the target gene and the larger fragment of the plasmid after digestion Use T4 DNA ligase for ligation reaction, transform the ligated product into competent cells of Escherichia coli DH5α strain, then spread the transformed competent cells on LB culture plates containing 50 μg / ml ampicillin, and culture overnight at 37°C .
[0064] Pick a single colony grown on the above-mentioned petri dish and inoculate it in LB liquid medium containing 50 μg / ml ampicillin, culture it with shaking at 37°C overnight, collect the bacteria for plasmid extr...
Embodiment 3
[0065] Example 3: Screening of Alcohol Dehydrogenase Mutants
[0066] The mutant in Example 1 was inoculated in 500 ml of LB liquid medium containing 50 μg / ml ampicillin, cultured with shaking at 37°C until OD600=0.6, added IPTG to a final concentration of 0.2mM, and induced expression at 18°C . After 16 hours of induction, the cells were collected by centrifugation at 6000g for 10 minutes. The cells were disrupted by an ultrasonic breaker (JY92-2D, Ningbo Xinzhi Biotechnology Co., Ltd.), and the supernatant was collected by centrifugation at 10,000 g at 4°C for 20 min to obtain a crude enzyme solution of alcohol dehydrogenase for activity detection. Add 50.0mg of main raw materials (tetrahydrofuro[2,3-b]furan-3(2H)-one, 5.0mg NAD+, 50.0mg ammonium formate, 10mg coenzyme formate dehydrogenase and 1.5ml alcohol dehydrogenase to a 10ml reaction vial Enzyme crude enzyme solution, system pH = 6.0, and after incubating at 30±3°C for 17 hours, extract the reaction system with dich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com