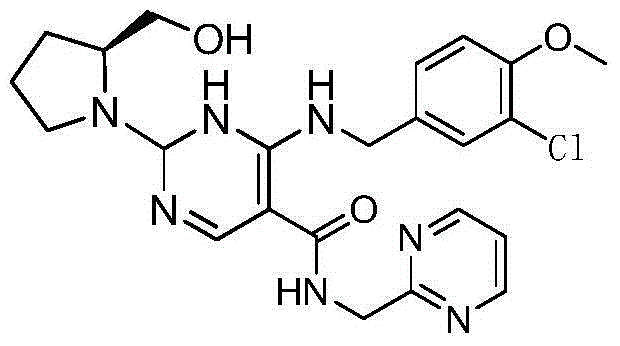
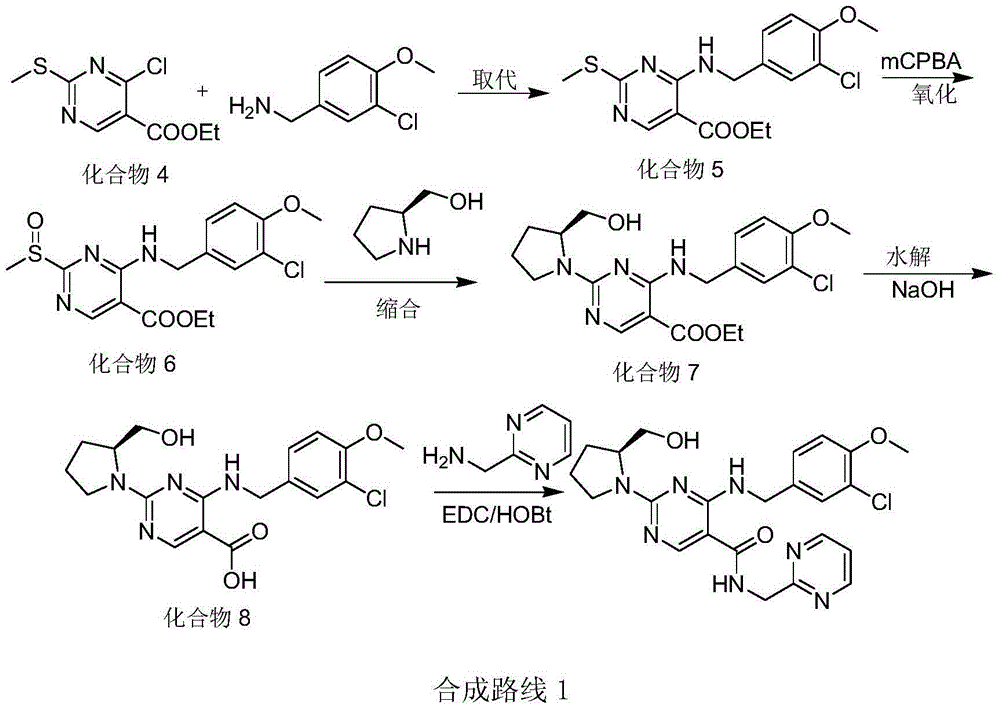
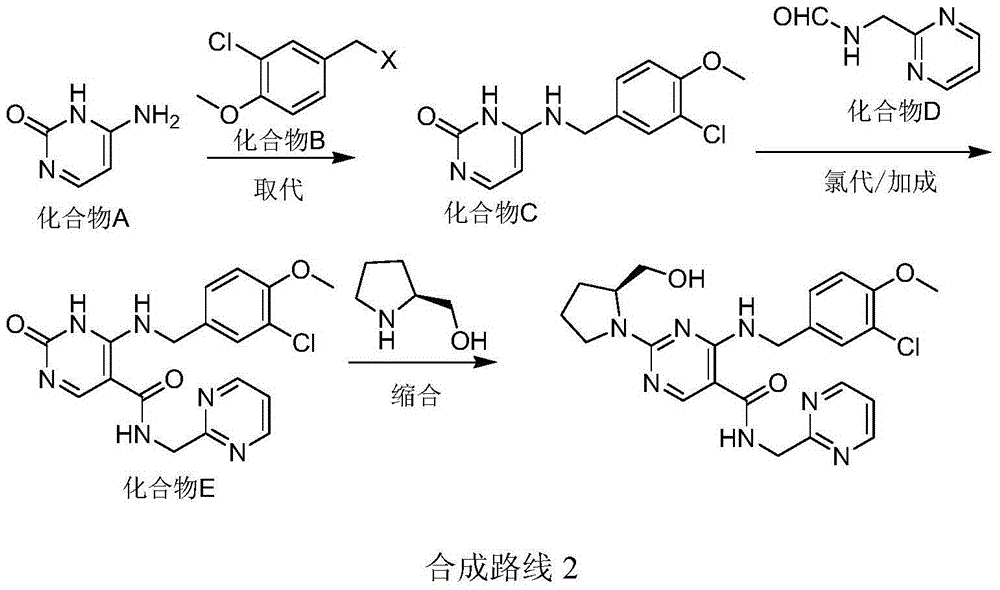
Preparation method of avanafil
A technology of avanafil and intermediates, applied in the field of medicine, can solve the problems of difficult industrialization, high industrialization cost, and low yield, and achieve the effects of easy industrialized production, low industrialized cost, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of 2-hydroxypyrimidine-4-carbamate isopropyl ester (intermediate I)
[0040] Dissolve 60g (0.540mol, M=111.1) cytosine in 600ml of dichloromethane, add 186.6g of potassium carbonate (1.35mol, M=138.21), then add di-tert-butyl dicarbonate (0.702 mol, M=218.25) in dichloromethane solution (dissolve 153.0g di-tert-butyl dicarbonate in 60ml dichloromethane), after the dropwise addition, keep warm for 1-2 hours, filter, and distill under reduced pressure to obtain an oily solid , intermediate I was prepared.
Embodiment 2
[0041] Embodiment 2: Preparation of ethyl 6-amino-2-hydroxypyrimidine-5-carboxylate (intermediate II)
[0042]Dissolve the intermediate I prepared in Example 1 in 450ml of hexamethyldisilazane, heat to 80-100°C for 1-3 hours, evaporate hexamethyldisilazane under reduced pressure; then add 360ml of di Chloromethane, after stirring evenly, add 62.4g of ethyl chloroformate (0.594mol, M=108.52) to cool down to 0-10°C, add 180.0g of aluminum trichloride (1.35mol, M=133.34) in batches, heat preservation reaction 1 -2 hours, then heat up to 20-30°C and keep it warm for 2-3 hours, add the feed solution dropwise to 600ml ice water containing 2% hydrochloric acid, stir for 0.5 hours, filter, and the filtrate is statically separated, and the water phase is 300ml×2 Extract with dichloromethane, combine the organic phases, wash twice with 300ml×2 5% sodium bicarbonate solution, dry over anhydrous sodium sulfate, filter, distill under reduced pressure, and dry to obtain 82.0g light yellow s...
Embodiment 3
[0043] Example 3: Preparation of ethyl 6-(3-chloro-4-methoxy-benzylamino)-2-hydroxypyrimidine-5-carboxylate (intermediate III)
[0044] The obtained 60g intermediate II (0.327mol) was dissolved in 300ml of dichloromethane, 90.5g of potassium carbonate (0.655mol, M=138.21) and 0.6g of potassium iodide (1%, M=138.21) were added, and then 81.8 g compound 2 (3.47mol, M=235.5), keep the temperature at 25-30°C for 3-4 hours, after the reaction is complete under TLC control, filter, the filtrate is distilled under reduced pressure, add 200ml of methanol and stir at 25-30°C for 0.5-1 hours, filtered, washed with 50ml of methanol, and dried to obtain 106.8g of intermediate III, with a yield of 96.5%; ESI (m / z): 338.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com