Preparation method of stable nano ferroferric oxide magnetofluid
A magnetic and nanotechnology of ferroferric oxide, which is applied in the direction of nanomagnetism, ferrous oxide, iron oxide/iron hydroxide, etc., can solve the problem of wide particle size distribution of nano ferroferric oxide and the stability of magnetic fluid aqueous solution low price, good crystal form, and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
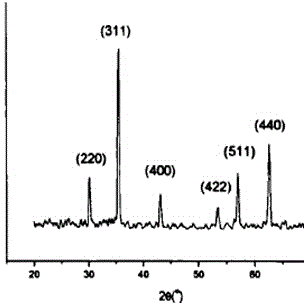
Image
Examples
Embodiment 1
[0027] The preparation of iron ferric oxide nanoparticles specifically comprises the following steps:
[0028] (a) Take 1 mL of ferric salt stock solution (4.5 M), add it to a 250 mL three-necked bottle, and dilute with distilled water;
[0029] (b) Take a certain amount of reductant vitamin C and EDTA disodium salt stock solution, add it to the ferric iron solution, control the ratio of reductant to ferric salt to 1:3, control EDTA The ratio of disodium acetate to ferric iron is 2:1;
[0030] (c) adjust the pH value to 11 with 1M sodium hydroxide solution;
[0031] (d) Introduce high-purity nitrogen gas into the solution for 30 minutes to remove the oxygen in the solution, so that the reaction proceeds under anaerobic conditions; heat to 70°C and reflux for 2 hours. In this example, the reaction solution turns black to prove that the obtained four Ferric oxide solution, if the solution is brown or reddish brown, it means that there is a part of ferric oxide in the solution....
Embodiment 2
[0036] The preparation of iron ferric oxide nanoparticles specifically comprises the following steps:
[0037](a) Take 1 mL of ferric salt stock solution (0.45 M), add it to a 250 mL three-necked bottle, and dilute with distilled water;
[0038] (b) Take a certain amount of reductant vitamin C and EDTA disodium salt stock solution, add it to the ferric iron solution, control the ratio of reductant to ferric salt to 1:3, control EDTA The ratio of disodium acetate to ferric iron is 2:1;
[0039] (c) adjust the pH value to 11 with 1M sodium hydroxide solution;
[0040] (d) Introduce high-purity nitrogen gas into the solution for 30 minutes to remove the oxygen in the solution, so that the reaction proceeds under anaerobic conditions; heat to 70°C and reflux for 2 hours. In this example, the reaction solution turns black to prove that the obtained four Ferric oxide solution, if the solution is brown or reddish brown, it means that there is a part of ferric oxide in the solution....
Embodiment 3
[0044] The preparation of iron ferric oxide nanoparticles specifically comprises the following steps:
[0045] (a) Take 1 mL of ferric salt stock solution (0.45 M), add it to a 250 mL three-necked bottle, and dilute with distilled water;
[0046] (b) Take a certain amount of stock solution of reducing agent hydrazine hydrate and 20kD dextran, add it to the ferric iron solution, control the ratio of reducing agent to ferric salt to 1:3.75, and control the amount of dextran to be 15 to 20g;
[0047] (c) adjust the pH value to 11.2 with 1M sodium hydroxide solution;
[0048] (d) Introduce high-purity nitrogen gas into the solution for 30 minutes to remove oxygen in the solution, so that the reaction proceeds under anaerobic conditions; heat to 70°C and reflux for 2 hours. In this case, the reaction solution turns black to prove that the tetraoxide is obtained Iron trioxide solution, if the solution is brown or reddish brown, it means that there is a part of ferric oxide in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com