Heavy metal ion adsorbent and preparation method thereof
A technology of heavy metal ions and adsorbents, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the problems of the ability to remove metal ions, affect the chelation of metal ions, etc., and achieve reuse rate High, easy to remove, good adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The invention also discloses a preparation method of a heavy metal ion adsorbent, comprising the following steps:
[0046] Ultrasonic dispersing the core of polyamide-amine dendrimers grafted by silane compounds in a buffer solution containing polyanions, and then standing still to obtain a heavy metal ion adsorbent;
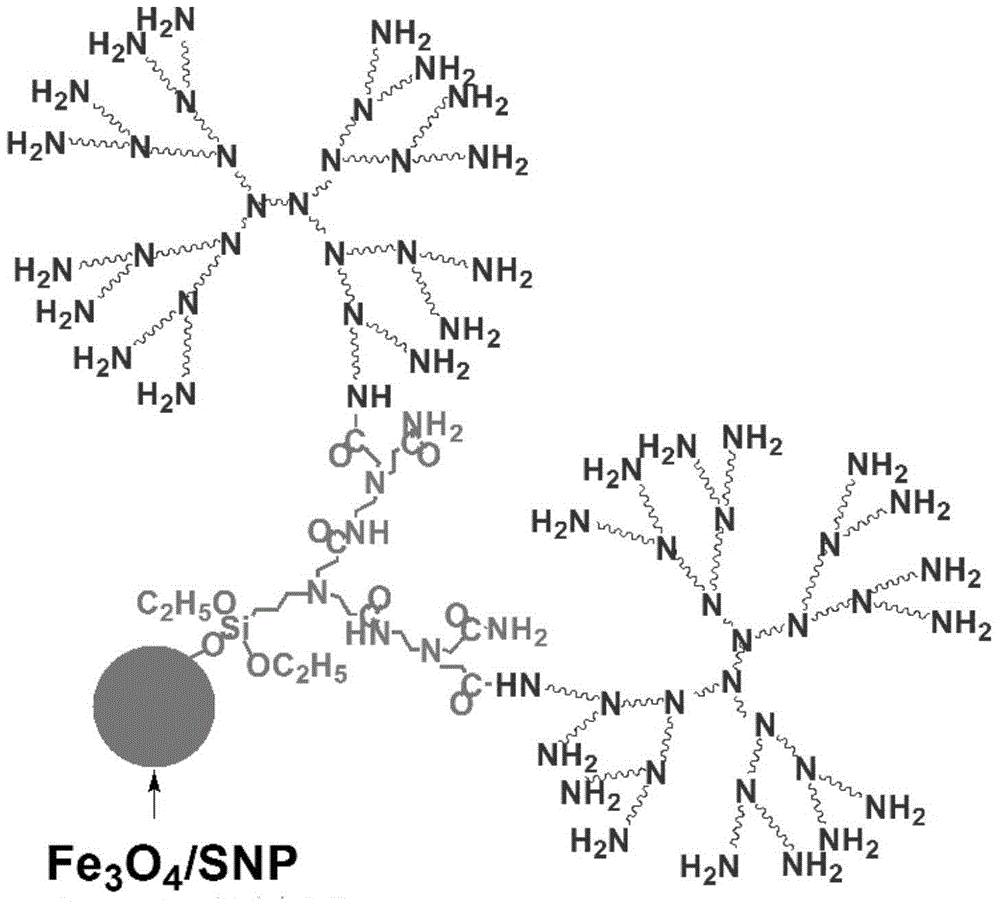
[0047] The core is ferric oxide coated with silicon dioxide.
[0048] The time for the ultrasonic dispersion is preferably 5 to 20 minutes, and the standing time is preferably 5 to 30 minutes,
[0049] According to the present invention, preferably, before the nucleus ultrasonic dispersion of grafted polyamide-amine dendrimers, it also includes: grafting the core and spatial structure of polyamide-amine dendrimers by silane compounds into: Spherical polyamidoamine dendrimers react in organic solvents in the dark. Through the light-shielding reaction, the polyamide-amine dendrimers with a spherical spatial structure are grafted on the polyamide-amine den...
Embodiment 1
[0066] At 25°C, 5.4g FeSO 4 ·7H 2 O and 6.8 g FeCl 3 (Molar ratio: Fe(III) / Fe(II)=2:1) was dissolved in 130 mL of high-purity argon-deoxygenated water, stirred and concentrated ammonia water (about 60 mL) was added dropwise until the pH was 10.0. Then, heat at 65° C. for 50 minutes, contact the outer wall of the container with a magnet to collect the magnetic nanomaterials, and wash the magnetic nanomaterials with deionized water for 3 times to neutralize the obtained black powder. Under nitrogen protection, 2 g of the synthesized magnetic Fe 3 o 4 Nanomaterials were dispersed in absolute ethanol, and 60 mL of ammonia water and 4 mL of tetraethyl orthosilicate (TEOS, Si(OC 2 h 5 ) 4 ). After continuing to sonicate in an ice-water bath for 2 h, the product was collected by contacting the container wall with a magnet, washed 3 times with absolute ethanol, and dried under vacuum (70 ° C, about 12 h) to obtain magnetic nanoparticles (Fe 3 o 4 SiO 2 ).
Embodiment 2
[0068] 0.5g Fe 3 o 4 SiO 2 Particles, 30mL toluene and 5mL 3-aminopropyltriethoxysilane were added into a 50mL flask, and the mixture was sonicated for 30min, then reacted at 110°C for 8h. After the reaction, the product was separated from the liquid with a magnet, washed five times with absolute ethanol (5 mL each time), and the obtained product was vacuum-dried at 50° C. for 24 h. Described product is 0 generation polyamido-amine graft (Fe 3 o 4 SiO 2 PADD G0).
[0069] 0.3g Fe 3 o 4 SiO 2 Add PADD G0 and 10mL methanol into the round bottom flask, sonicate for 30min to make the Fe 3 o 4 SiO 2 PADD G0 was dispersed, then 0.1mL methyl acrylate was slowly added into the flask, and reacted at 50°C for 48h. After the reaction, use a magnet to separate the product from the liquid, wash it with anhydrous methanol 5 times (2 mL each time), and dry the product in vacuum at 50 °C for 24 hours. The product is Fe 3 o 4 SiO 2 PADD G0.5.
[0070] 0.3g Fe 3 o 4 SiO 2 Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com