Novel chemotherapeutic drug nano ADC based on antibody conjugation and preparation method and application thereof
A technology of chemotherapeutic drugs and conjugates, applied in the field of medicine, can solve problems affecting the effective combination of antibodies and antigens, reduce killing, and reduce ADC activity, and achieve the effects of improving bioavailability, good targeting effect, and enhancing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
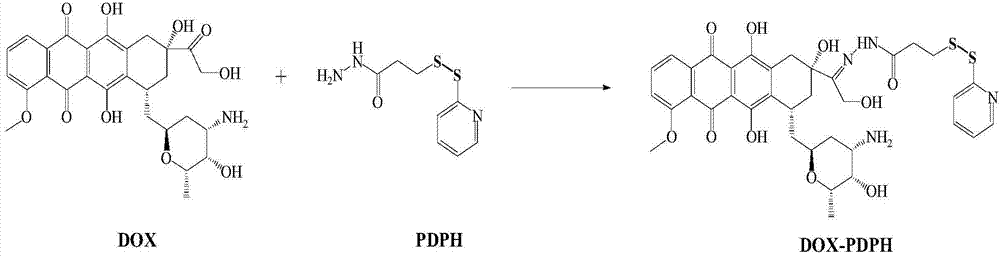
[0048] Embodiment 1 has the synthesis of disulfide bond doxorubicin (DOX-PDPH)
[0049] Weigh 27.25 mg of doxorubicin (ADR), 11.78 mg of hydrazino-4-(2-pyridinedithio)butanoic acid (PDPH), dissolve in 3 ml of methanol; stir and react in the dark at room temperature for 6 days , react at different stirring speeds (100rpm, 500rpm, 1000rpm, 3000rpm); fast halving speed can prevent the precipitation of doxorubicin, thereby facilitating the synthesis of ADR-PDPH. After the reaction, an orange-yellow product can be obtained. The schematic diagram of the synthesis of ADR-PDPH is shown in figure 1 .
Embodiment 2
[0050] Example 2 Preparation of mercaptolated Her2
[0051] According to the ratio of 1 mg of her2 and 75 μg 2-IT (2-iminothiolane HCI (Traut's reagent, Shanghai Qianchen Biotechnology Co., Ltd.)) (the molar ratio of Anti-Her2-Fab and 2-IT is 1:100 or according to the actual The situation increases the ratio of the two) to mix the two. Seal with parafilm to prevent 2-IT from being oxidized. Reaction at room temperature for 2h. The reaction system can be scaled up. The thiolated Her2 was dialyzed (PBS, 5 mM EDTA, pH=7.4) to remove excess Traut's reagent. Change the dialysate every 6-8 hours, and dialyze 2-3 times.
Embodiment 3
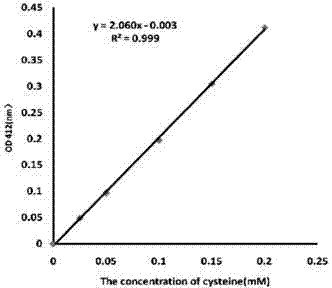
[0052] Example 3 Antibody thiolation standard curve
[0053] Ellman's test measures the -SH amount of thiolated antibody, and DTNB is Ellman's reagent. It is used for the colorimetric determination of sulfhydryl groups in biological samples. It is easily soluble in water. In the presence of mercapto compounds, colorless DTNB will be transformed into yellow 5-mercapto-2-nitrobenzoic acid. Since 5-mercapto-2-nitrobenzoic acid has the maximum absorption at 412nm, the absorption spectrum of DTNB does not interfere with the determination of sulfhydryl.
[0054] Reagent configuration:
[0055] (1) Tris-HCl buffer solution (0.25M): After accurately preparing DDW (deuterium depleted water), adjust the pH to 8.3 with hydrochloric acid, and configure 300ml.
[0056] (2) Cysteine standard solution (1mM): Accurately weigh 0.017563g of L-cysteine (molecular weight: 175.63), dissolve it in 1ml of formic acid, and dilute to 100ml with DDW.
[0057] (3) DTNB (molecular weight: 396.35...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com