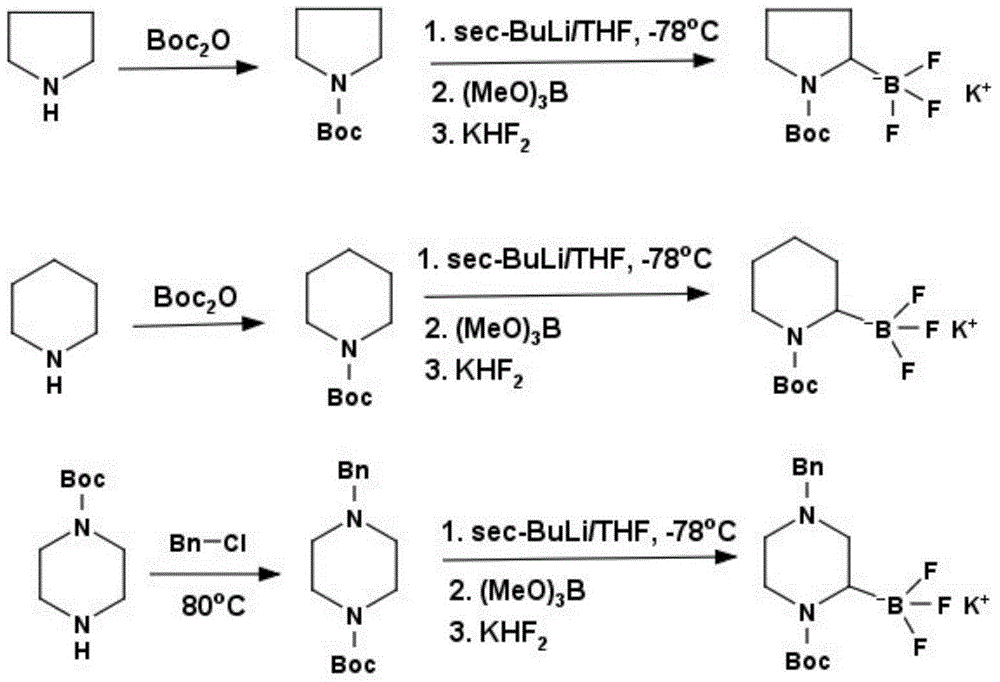
Method for preparing saturated potassium azacyclo-trifluoroborates
A technology of potassium trifluoroborate and potassium tetrahydropyrrole trifluoroborate, which is applied in the field of organic chemical synthesis, can solve the problems of difficult organic chemical reagents, unstable boric acid, and difficulty in preparation, and achieves simple and easy preparation process, Easy to commercialize product, good purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of N-tert-butylcarbonyltetrahydropyrrole:
[0022] In a 3L reaction flask, add 100g of tetrahydropyrrole (1.41mol), 1L of tetrahydrofuran and 8.58g of 4-dimethylaminopyridine (0.07mol), cool to 0°C, and slowly add 307.73g of tert-butylcarbonic anhydride (1.41mol) After the addition, it was raised to room temperature, reacted for 12 hours, added water to dilute, extracted with ether, separated, dried, and evaporated the solvent to obtain 221 g of N-tert-butylcarbonyl tetrahydropyrrole, with a yield of 92%, 1H NMR ( CDCl 3 ): 1.21ppm, unimodal (9H); 1.78ppm, multimodal (4H); 3.21ppm, multimodal (4H).
Embodiment 2
[0024] Preparation of N-tert-butylcarbamoylpiperidine:
[0025] In a 3L reaction flask, add 100g of piperidine (1.17mol), 1L of tetrahydrofuran and 7.17g of 4,4-dimethylaminopyridine (0.06mol), cool to 0°C, and slowly add 255.35g of tert-butylcarbonic anhydride (1.17mol ), the addition was completed, raised to room temperature, reacted for 12 hours, added water to dilute, extracted with ether, separated, dried, and evaporated the solvent to obtain 210 g of N-tert-butylcarbonylpiperidine, with a yield of 96%, 1H NMR ( CDCl 3 ): 1.26ppm, unimodal (9H); 1.45ppm, multimodal (4H); 1.68ppm, multimodal (2H); 13.18ppm, multimodal (4H).
Embodiment 3
[0027] Preparation of N-tert-butylcarbonyl-4-benzylpiperazine:
[0028] In a 5L reaction flask, add 150g N-tert-butylcarboylpiperazine (0.81mol), 107.04g benzyl chloride (0.85mol), 222.62g potassium carbonate (1.61mol) and 2L ethanol, heat to reflux for 12 hours, cool to At room temperature, filter, concentrate the solvent, add water to dilute, extract with dichloromethane, separate the organic phase, wash with water, dry, concentrate the solvent to obtain a crude product, recrystallize with n-hexane to generate N-tert-butylcarbonyl-4-benzyl Piperazine 190g, productive rate 85%, 1H NMR (CDCl 3 ): 141ppm, unimodal (9H); 2.40ppm, multimodal (4H); 3.21ppm, multimodal (4H); 3.26ppm, unimodal (2H); 7.28ppm, multimodal (5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com