Compounds as modulators of a mutant CFTR protein and their use for treating diseases associated with CFTR protein malfunction
A technology of protein abnormalities and compounds, applied in the fields of compounds of group 5/15 elements of the periodic table, active ingredients of heterocyclic compounds, diseases, etc., can solve problems such as exocrine pancreatic duct obstruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
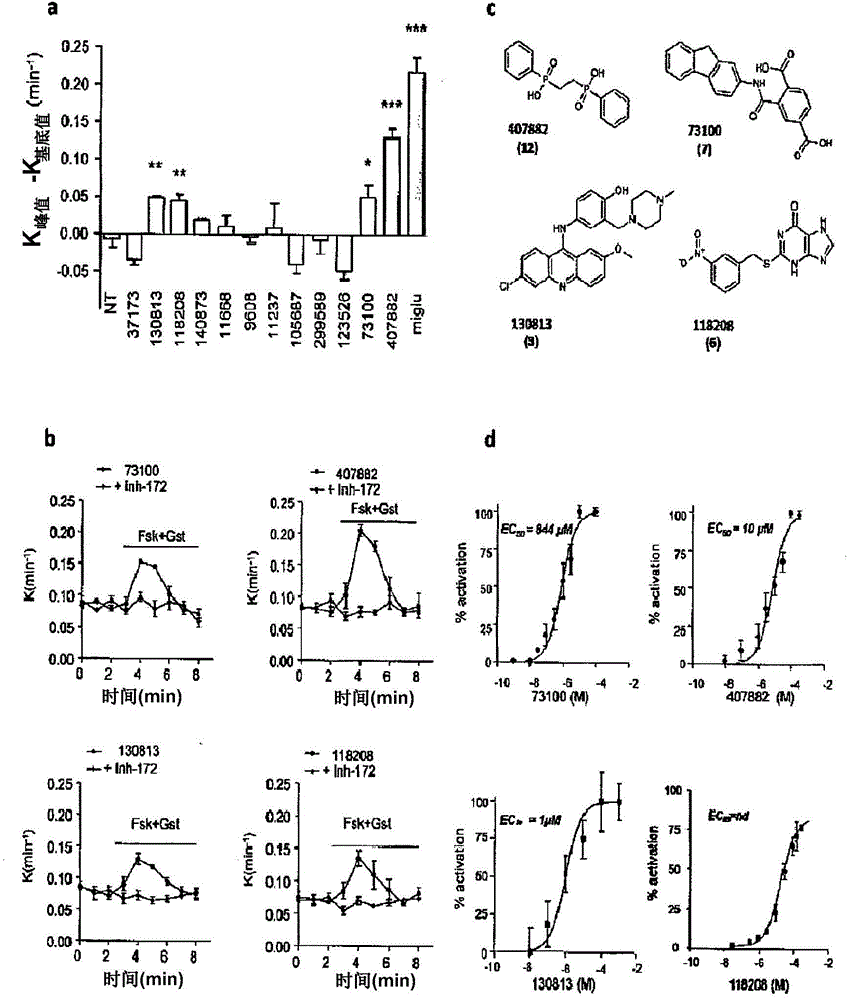
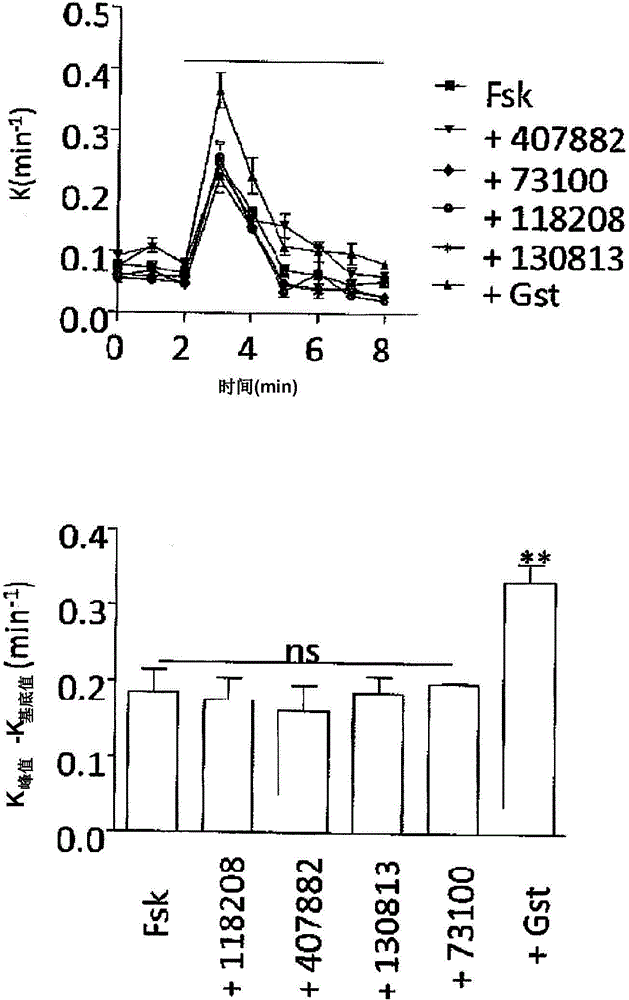
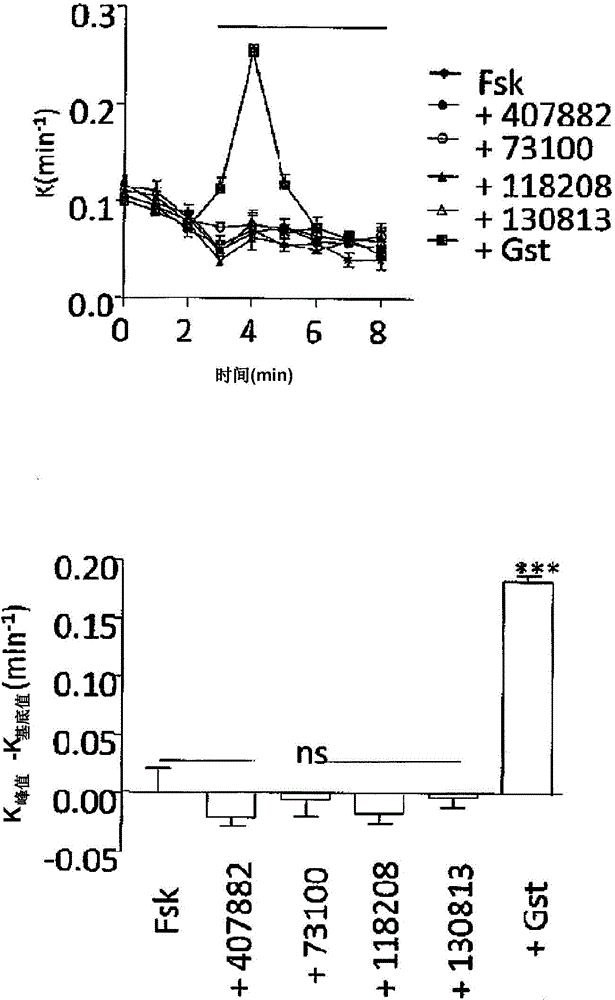
Image
Examples
Embodiment
[0102] Materials and methods
[0103] Antibody
[0104] The following antibodies were used: MAB25031 (clone 24-1, R&D systems, USA) and MM13-4 (Upstate), monoclonal antibodies (mAb) for CFTR detection; fluorescent secondary antibodies Alexa594 and 488 from Molecular Probes (Cergy Pontoise, France )Buy.
[0105] cell culture
[0106] Stably transfected HeLa cells (HeLa cells only contain the pTracer plasmid as a control (pTracer), or express WT-CFTR (spTCF-WT), ΔF508-CFTR (spTCF-F508del)) were prepared by Pascale Fanen (Inserm U.468, Créteil , France) and cultured as described elsewhere (16). Briefly, HeLa cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated FCS, 100 U / ml penicillin, 100 μg / ml streptomycin, and 250 μg / ml zeocin antibiotics. at 37°C in 5% CO 2 cultured in a humidified incubator. Expression of WT-CFTR and ΔF508-CFTR in these cells was verified by immunoprecipitation and immunocytochemistry throughout t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com