Preparation method of chlorine trifluoride
A technology of chlorine trifluoride and fluorine gas, which is applied in the direction of interhalogen compounds, etc., can solve the problems of low reliability of equipment, large storage and transportation of raw material chlorine gas, complicated production process, etc., and achieve convenient feeding and discharging, cheap raw materials, The effect of simple reactor structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
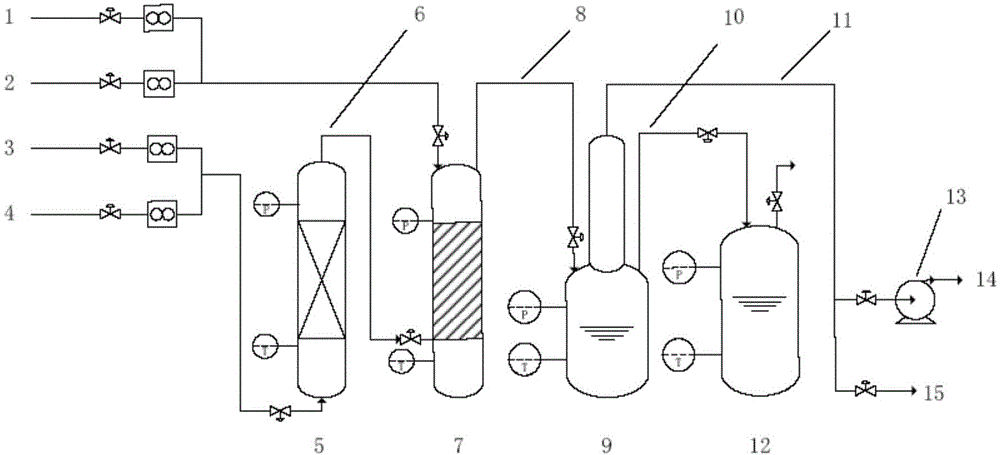
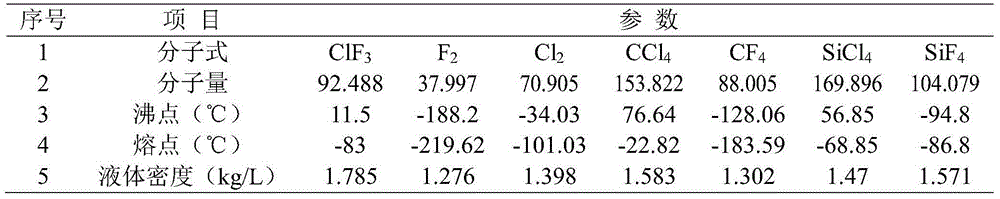
[0052] (1) Firstly, the vacuum pump is used to vacuumize the various parts of the device; then use fluorine gas to purge and replace the residual air and moisture in the system; then excessive CCl 4 Add in the gas-liquid reactor, and feed fluorine gas from the bottom of the gas-liquid reactor at a flow rate of 0.1L / min, CCl 4 Bubble reaction with fluorine gas at 0.6MPa and 50°C to obtain product 1;
[0053] (2) Feed product 1, fluorine gas and nitrogen into the synthesis reactor with the flow velocity of 0.1L / min, 0.3L / min and 1.2L / min respectively, set the operating pressure of the synthesis reactor to be 0.6MPa, at 100 Reaction at ℃ to obtain product 2;
[0054] (3) Pass the product 2 into the cold trap, set the operating pressure of the cold trap to 0.6MPa, liquefy the product 2 at 0°C, and send the low-boiling point gas impurities to the tail gas treatment system through the exhaust pipeline. Empty after chemical treatment. After 20 hours, the feeding of raw gas was sto...
Embodiment 2
[0059] (1) First, the vacuum pump is used to pre-treat the various parts of the device; then nitrogen is used to purge and replace the residual air and moisture in the system; then the excess SiCl 4 Add in the gas-liquid reactor, and feed fluorine gas from the bottom of the gas-liquid reactor at a flow rate of 1L / min, SiCl 4 Bubble reaction with fluorine gas at 0.03MPa and 25°C to obtain product 1;
[0060] (2) Pass the product 1, fluorine gas and nitrogen gas into the synthesis reactor at a flow rate of 1L / min, 4L / min and 40L / min respectively, set the operating pressure of the synthesis reactor to 0.32MPa, and react at 380°C , to obtain the product 2;
[0061] (3) Pass the product 2 into the cold trap, set the operating pressure of the cold trap to 0.05MPa, liquefy the product 2 at -95°C, and send the low-boiling point gas impurities to the tail gas treatment system through the exhaust pipeline. Void after chemical treatment. After 3 hours, the feeding of raw material gas ...
Embodiment 3
[0066] (1) First, carry out vacuum pretreatment on each part of the device by a vacuum pump; then use nitrogen to purge and replace the residual air and moisture in the system; then excessive CCl 4 and SiCl 4 mixed solution (CCl 4 with SiCl 4 The volume ratio is 2:1) into the gas-liquid reactor, and fluorine gas, CCl 4 、SiCl 4 Bubble reaction with fluorine gas at -0.09MPa, -20°C to obtain product 1;
[0067] (2) Feed product 1, fluorine gas and nitrogen into the synthesis reactor with the flow rate of 0.5L / min, 1L / min and 0.51L / min respectively, set the operating pressure of the synthesis reactor to be-0.09MPa, at 400 Reaction at ℃ to obtain product 2;
[0068] (3) Pass the product 2 into the cold trap, set the operating pressure of the cold trap to -0.09MPa, liquefy the product 2 at -100°C, and send the low-boiling point gas impurities to the tail gas treatment system through the exhaust pipeline. Release it after harmless treatment. After 10 hours, the feeding of raw ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com