Nonaqueous electrolyte battery
A non-aqueous electrolyte and battery technology, which is applied in the manufacture of non-aqueous electrolyte batteries, electrodes of non-aqueous electrolyte batteries, and electrolyte batteries, and can solve problems such as side reactions and capacity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0016] Embodiments provide a nonaqueous electrolyte battery. The nonaqueous electrolyte battery includes a positive electrode, a negative electrode, and a nonaqueous electrolyte. The positive electrode includes a positive electrode current collector and a positive electrode active material-containing layer formed on the positive electrode current collector. The positive electrode active material-containing layer contains at least one lithium-nickel composite oxide and a conductive agent. In the particle size distribution obtained by the laser diffraction scattering method of the layer containing the positive electrode active material, the average particle diameter d 50 In the range of 1 μm to 5.5 μm, the maximum particle size is in the range of 10 μm to 100 μm, and the cumulative frequency from the small particle size side is 10% particle size d 10 It is in the range of 0.5 μm to 3 μm. By X=(d 50 -d 10 ) / d50 is in the range of 0.5 or more and less than 1.
[0017] The li...
Embodiment 1
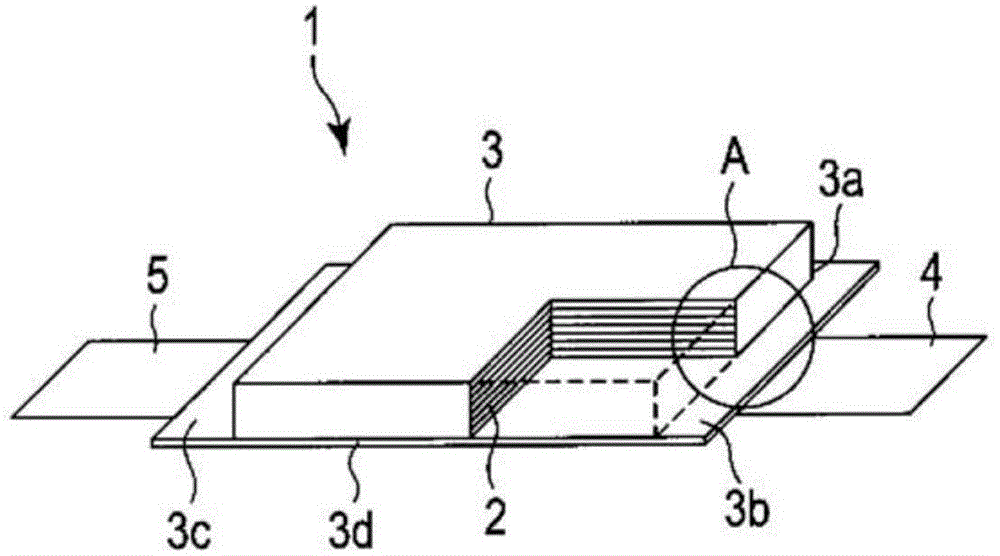
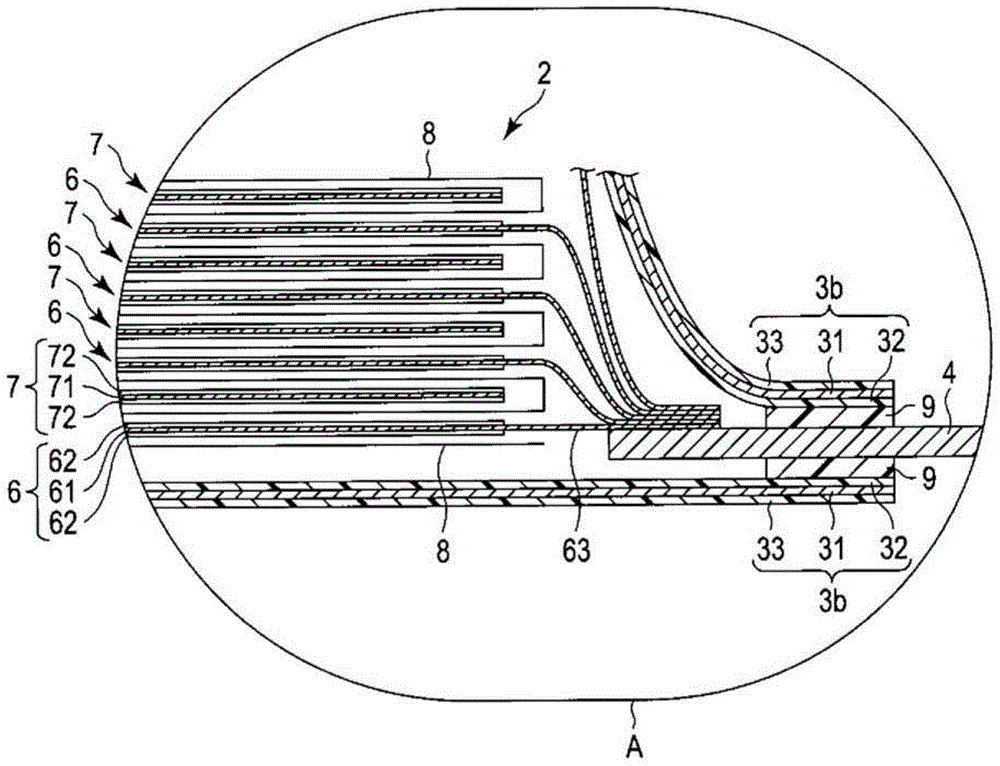
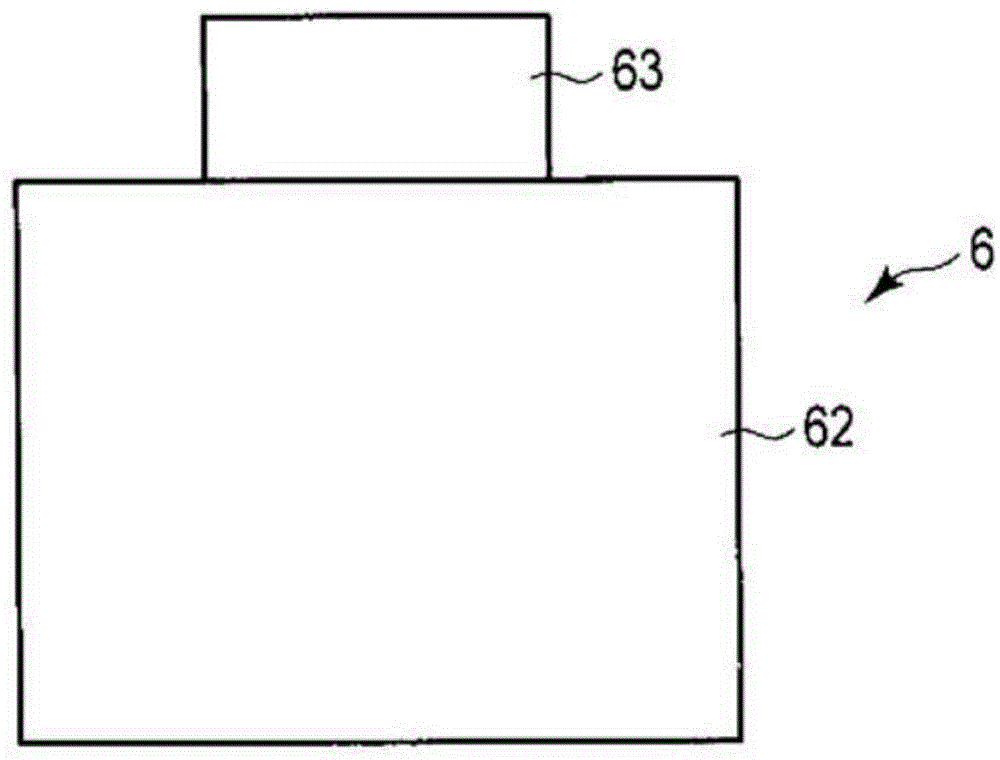
[0118] In Example 1, follow the steps below to make Figure 1 ~ Figure 3 The non-aqueous electrolyte battery 1 shown.
[0119] [Production of positive electrode 6]
[0120] As the positive electrode active material, LiNi with an average particle size of 6 μm is used 7 / 10 co 2 / 10 mn 1 / 10 o 2 . The active material, acetylene black, graphite and polyvinylidene fluoride were mixed in a ratio of 100:8:5:3 according to the following steps. First, the active material, acetylene black and graphite are dry mixed using a Henschel mixer. After dry mixing, polyvinylidene fluoride and N-methyl-2-pyrrolidone were added to the obtained dry mixture, and wet mixing was performed with a planetary mixer. Thereby, a mixture containing each material in the above ratio was produced.
[0121] Next, the obtained mixture was put into an autorotation-revolution mixer THINKY Seinataro (ARE-250), and the rotation speed was set at 2000 rpm, and stirring was performed for 30 minutes. Next, the mix...
Embodiment 2
[0146] In Example 2, the nonaqueous electrolyte battery 1 was produced in the same procedure as in Example 1 except that the rotational speed of the sand mill was set to 1000 rpm.
[0147] Regarding the non-aqueous electrolyte battery 1 of Example 2, the charge-discharge cycle characteristics and particle size distribution were evaluated in the same manner as in Example 1.
[0148] The capacity retention rate after 300 cycles of the non-aqueous electrolyte battery 1 of Example 2 was 85%. In addition, in the particle size distribution related to the non-aqueous electrolyte battery 1 of Example 2, the average particle size d 50 3.9μm, particle size d 10 is 1.37 μm, the maximum particle size is 15.4 μm, and the value of X is 0.65.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com