Manidipine hydrochloride tablets and preparation method thereof
A technology of manidipine hydrochloride and manidipine hydrochloride, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as unfavorable absorption and degradation, and achieve improved dissolution rate, good stability, and preparation The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
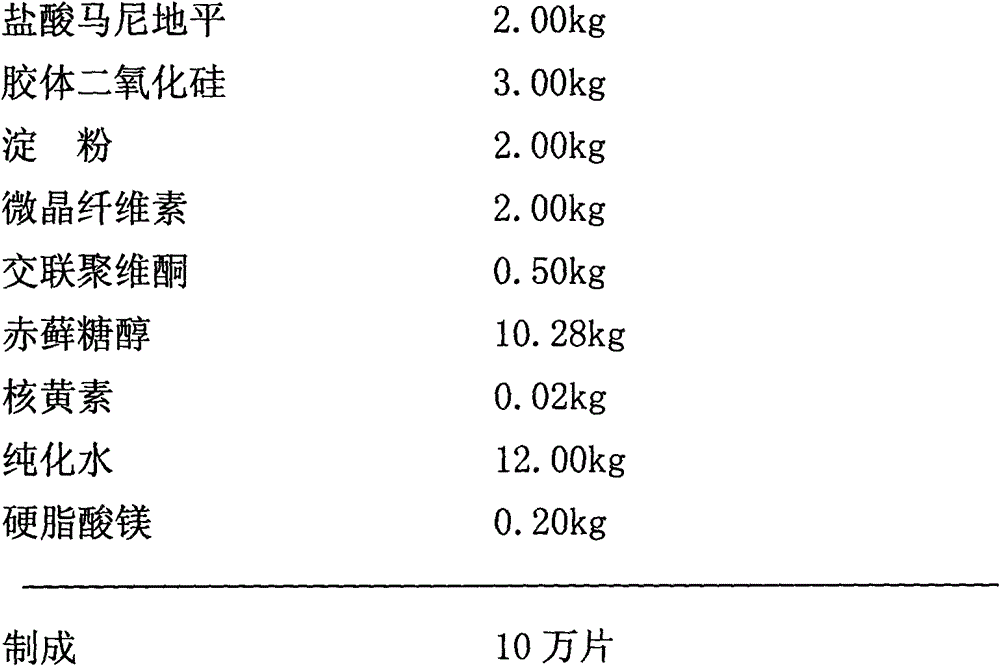
[0027] Embodiment 1: manidipine hydrochloride tablet (specification 20mg)
[0028] prescription:
[0029]
[0030] The above tablets are prepared according to the following method: first disperse manidipine hydrochloride, half the amount of colloidal silicon dioxide and crospovidone in water; then add the remaining colloidal silicon dioxide, starch , microcrystalline cellulose, erythritol and riboflavin, mixed evenly, and granulated by wet method; the wet granule material was dried and sieved; magnesium stearate was added to the sieved dry granules and mixed evenly ; Finally, the resulting mixture is compressed into manidipine hydrochloride tablets.
Embodiment 2
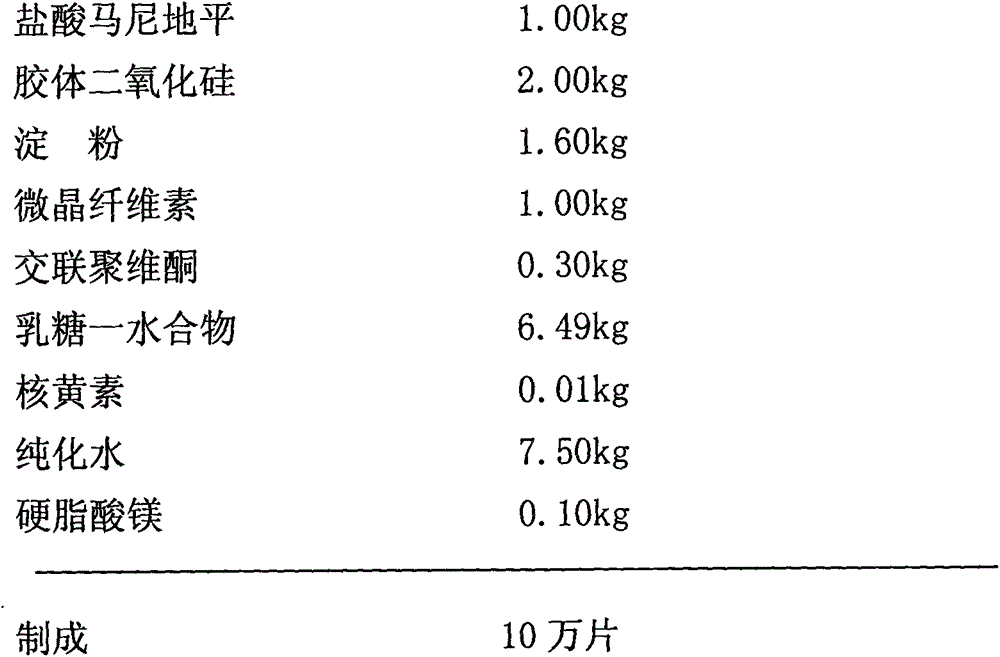
[0031] Embodiment 2: manidipine hydrochloride tablet (specification 10mg)
[0032] prescription:
[0033]
[0034] The above tablets are prepared according to the following method: first disperse manidipine hydrochloride, half the amount of colloidal silicon dioxide and crospovidone in water; then add the remaining colloidal silicon dioxide, starch , microcrystalline cellulose, lactose monohydrate and riboflavin, mix well, and use wet granulation; dry the wet granule material and sieve; add magnesium stearate to the sieved dry granule and mix uniform; finally the resulting mixture is compressed into manidipine hydrochloride tablets.
Embodiment 3
[0035] Embodiment 3: the preparation of manidipine hydrochloride tablet (specification 5mg)
[0036] prescription:
[0037]
[0038] The above tablet was prepared according to the following method:
[0039] First disperse manidipine hydrochloride, half of the amount of colloidal silicon dioxide and crospovidone in water; then add the remaining colloidal silicon dioxide, starch, microcrystalline cellulose, lactose to the formed suspension Hydrate and riboflavin, mixed uniformly, and wet granulated; the wet granulated material was dried and sieved; magnesium stearate was added to the sieved dry granules and mixed uniformly; Nidipine tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com