Method for synthesizing aromatase inhibitor
An aromatase and inhibitor technology, applied in the field of drug synthesis, can solve the problems of incomplete bromination reaction, unfavorable industrial production, low product content, etc., and achieve the effects of mild reaction conditions, good quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
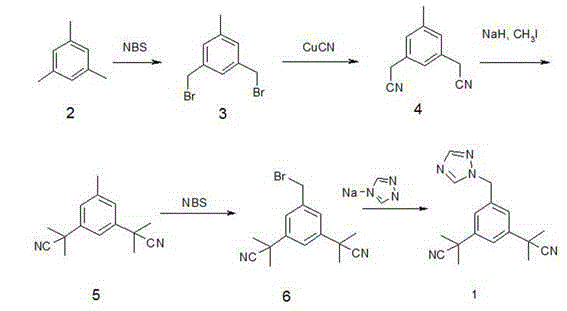
[0026] 1. Synthesis of intermediate A1: methyl 3,5-dibromomethylbenzoate:
[0027] Add 82g (0.5 mol) of methyl 3,5-dimethylbenzoate, AIBN (0.5g) and acetonitrile (500mL) into the reaction flask, add 180g (1.0mol) of NBS, stir and heat to reflux 75~80°C, Stir for 3 hours, the reaction is complete, concentrate under reduced pressure below 60°C, cool to room temperature, add 250mL of dichloromethane, wash with 50mL of 10% sodium sulfite solution, 50mL of 10% sodium carbonate solution, and 50mL of purified water, and dry over anhydrous sodium sulfate for 35 Concentrate at -40°C to obtain a yellow oil. Recrystallized with 300 mL of absolute ethanol to obtain 90 g of white solid crystals, yield 85.0%, mp 99-101 °C. 1H-NMR (CDCl 3 ) δ : 3.8 9(3H, s, OCH 3 ), 4 .25 (4H,s,CH 2 ), 7. 60(1H,t , J = 1.8 Hz,Ar-H), 8.02(2H,d , J =1 .8 Hz,Ar-H) .
[0028] 2. Synthesis of intermediate A2: methyl 3,5-dicyanomethylbenzoate:
[0029] Dissolve 80g (0.25mol) of methyl 3,5-dibromomethylbenzoa...
Embodiment 2
[0037] Synthesis of Anastrozole:
[0038] Add 48g (0.2mol) of intermediate 3,5-bis[(2,2-dimethyl)cyanomethyl]benzaldehyde and 13.0g (0.2mol) of 1,2,4-triazole to 200 mL of tetrahydrofuran Warm stirring for 2 hours, adding 42 g (0.2 mol) of sodium triacetate borohydride, stirring and heating to 45~55 ° C for 5 hours. After the reaction is complete, distill off THF under reduced pressure, pour the reaction solution into 200 mL of water, extract with ethyl acetate (200 mL×3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and distill off the solvent under reduced pressure to obtain a yellow oil The product was recrystallized from 200 mL of isopropanol to obtain 54 g of a white solid, with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com