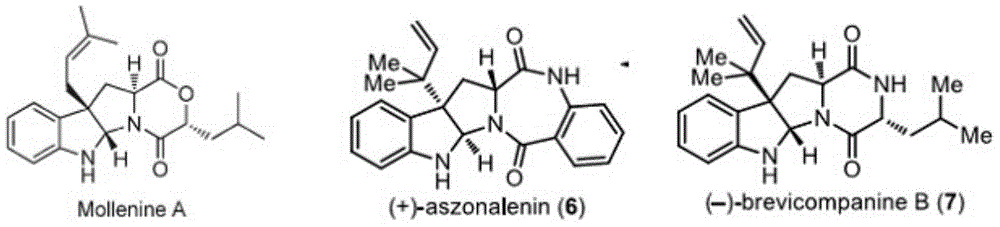
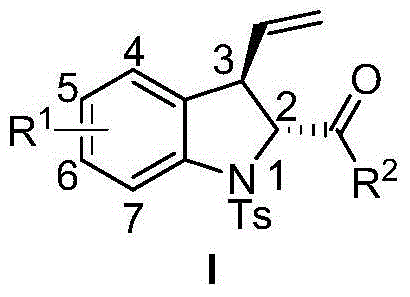
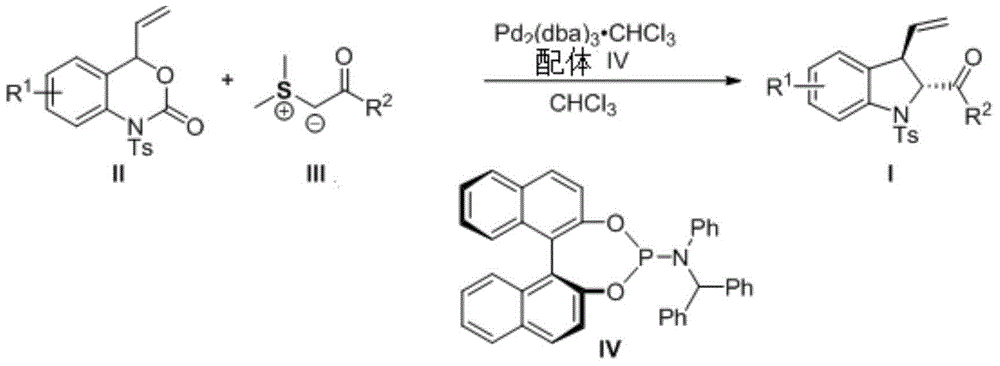
3-vinyl indoline derivatives with optical activity and asymmetric synthesis method of same
A technology based on indolines and optical activity, applied in the field of 3-vinyl indoline derivatives and their asymmetric synthesis, can solve the problems of high operating cost, large functional group limitations, cumbersome steps, etc., and achieve high The effect of enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Compound I-1
[0027] preparation of
[0028] At room temperature, the metal catalyst tris(dibenzylideneacetone)dipalladium chloroform adduct (10.35mg, 0.01mmol) and ligand IV (12.6mg, 0.022mmol) were dissolved in chloroform and stirred for 20 minutes under nitrogen protection , and then cooled to -40 degrees Celsius. Then add 4-vinylbenzoxazinone II-1 (66mg, 0.2mmol), continue to stir at -40 degrees Celsius for 5 minutes, then add sulfur ylide III-1 (54mg, 0.3mmol) to the reaction system , the reaction mixture continues to react at -40°C until TLC detects that the reaction is complete, with V 石油醚 / V 乙酸乙酯 =30:1-12:1 Column chromatography directly obtained 80.7 mg of the target product of formula I-1, with a yield of 99%.
[0029] 1 H NMR (600MHz, CDCl 3 )δ (ppm) = 8.00 (d, J = 7.5Hz, 2H), 7.75 (d, J = 8.0Hz, 2H), 7.62 (t, J = 9.6Hz, 2H), 7.50 (t, J = 7.6Hz ,2H),7.27(d,J=7.8Hz,3H),7.02(t,J=7.2Hz,1H),6.97(d,J=7.4Hz,1H),5.44–5.35(m,1H),5.32 (d, J = 4.9Hz, 1H), 4....
Embodiment 2
[0036] Compound I-2
[0037] preparation of
[0038] At room temperature, metal catalyst tris(dibenzylideneacetone)dipalladium chloroform adduct (10.35mg, 0.01mmol) and ligand IV (12.6mg, 0.022mmol) were dissolved in chloroform, under nitrogen protection Stir for 20 minutes, then cool down to -40 degrees Celsius. Then add 4-vinylbenzoxazinone II-1 (66mg, 0.2mmol), continue to stir at -40 degrees Celsius for 5 minutes, then add sulfur ylide III-2 (63mg, 0.3mmol) to the reaction system , the reaction mixture continues to react at -40°C until TLC detects that the reaction is complete, with V 石油醚 / V 乙酸乙酯 =30:1-12:1 column chromatography directly obtained 86.6 mg of the target product of formula I-2, with a yield of 99%.
[0039] 1 H NMR (600MHz, CDCl 3)δ (ppm) = 7.98 (d, J = 8.5Hz, 2H), 7.74 (d, J = 7.9Hz, 2H), 7.64 (d, J = 8.0Hz, 1H), 7.25 (d, J = 7.2Hz ,3H),7.03–6.97(m,1H),6.95(d,J=8.3Hz,3H),5.39-5.33(m,1H),5.29(d,J=4.7Hz,1H),5.00-4.92( m,2H), 3.85(s,3H), 3.79-3.76(m,1...
Embodiment 3
[0046] Compound I-3
[0047] preparation of
[0048] At room temperature, metal catalyst tris(dibenzylideneacetone)dipalladium chloroform adduct (10.35mg, 0.01mmol) and ligand IV (12.6mg, 0.022mmol) were dissolved in chloroform, under nitrogen protection Stir for 20 minutes, then cool down to -40 degrees Celsius. Then add 4-vinylbenzoxazinone II-1 (66mg, 0.2mmol), continue to stir at -40 degrees Celsius for 5 minutes, then add sulfur ylide III-3 (60mg, 0.3mmol) to the reaction system , the reaction mixture continues to react at -40°C until TLC detects that the reaction is complete, with V 石油醚 / V 乙酸乙酯 =30:1-12:1 column chromatography directly obtained 74.6 mg of the target product of formula I-3, with a yield of 92%.
[0049] 1 H NMR (600MHz, CDCl 3 )δ (ppm) = 7.90 (d, J = 7.2Hz, 2H), 7.74 (d, J = 7.2Hz, 2H), 7.64 (d, J = 7.9Hz, 1H), 7.29-7.25 (m, 5H) ,7.04–6.92(m,2H),5.41-5.37(m,1H),5.32(s,1H),4.97(dd,J=21.5,13.4Hz,2H),3.78(s,1H),2.42(s ,3H), 2.38(s,3H).
[0050] 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com