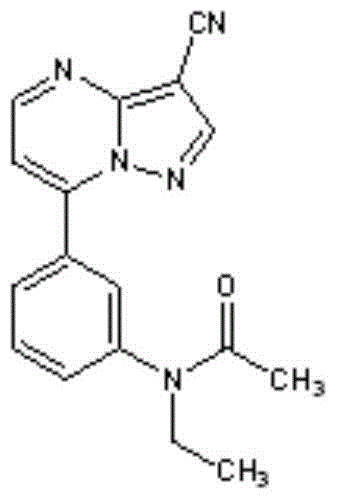
PH-independent zaleplon dipulse release capsule and method for preparing same
A zaleplon, double-release technology, applied in the field of pH-independent double-pulse release capsules and its preparation, can solve the problems of drowsiness, dizziness, inconvenience to patients, and affect the effectiveness of long-release dosage forms, so as to prolong sleep duration Time, increase sleep time, reduce the effect of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: pH-independent zaleplon double-pulse release capsules and preparation method thereof
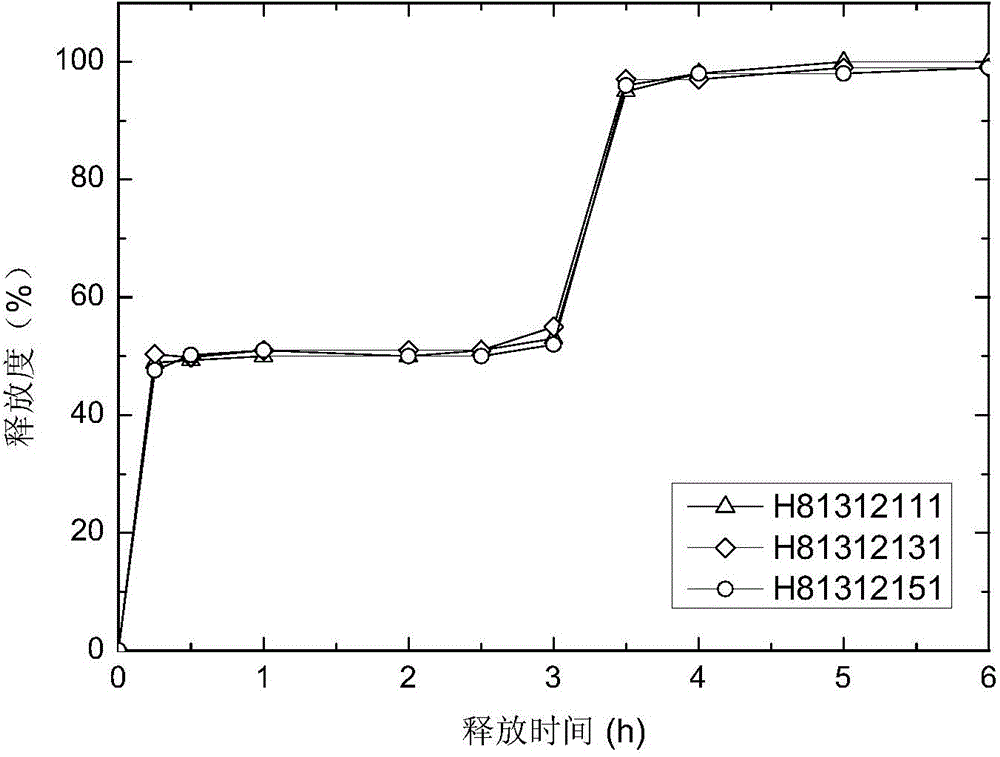
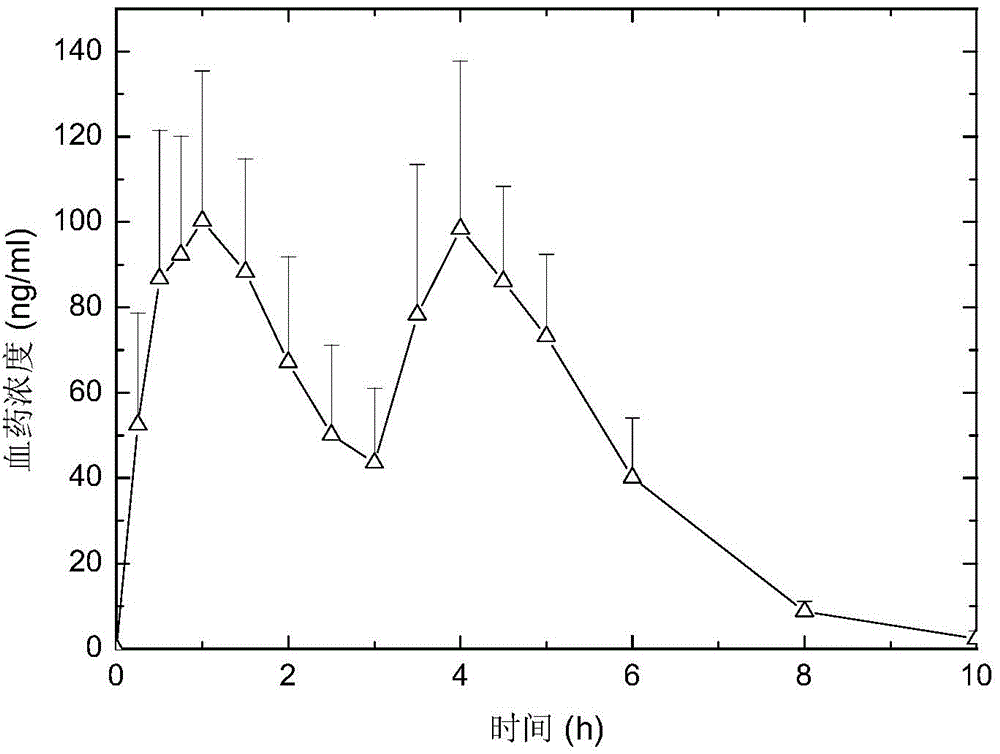
[0079] The Zaleplon double-pulse release capsule of the present invention comprises an immediate-release tablet and a timed-release tablet, and the weight ratio of the active ingredient zaleplon contained in the immediate-release tablet and the timed-release tablet is 1:1,
[0080] The preparation method of zaleplon double-pulse release capsules, the preparation method first adopts the tablet press to compress the immediate-release tablet according to the commonly used techniques and methods in pharmacy; according to the formula, the tablet press is used to compress the drug-containing tablet core, and the drug-containing tablet core Use a coating pan to coat, and the coating film is attached to the drug-containing tablet core to obtain a time-release tablet; put the above-prepared immediate-release tablet and time-release tablet into a capsule to obtain zaleplon double puls...
Embodiment 2
[0091] Example 2: pH-independent zaleplon double-pulse release capsules and preparation method thereof
[0092] The zaleplon double-pulse release capsule of the present invention, the weight ratio of the active ingredient zaleplon contained in the immediate-release tablet and the time-selected release tablet is 1:1,
[0093] The preparation method of zaleplon double-pulse release capsules, the preparation method first adopts the tablet press to compress the immediate-release tablet according to the commonly used techniques and methods in pharmacy; according to the formula, the tablet press is used to compress the drug-containing tablet core, and the drug-containing tablet core Use a coating pan to coat, and the coating film is attached to the drug-containing tablet core to obtain a time-release tablet; put the above-prepared immediate-release tablet and time-release tablet into a capsule to obtain zaleplon double pulse Release the capsule.
[0094] Basically the same as Examp...
Embodiment 3
[0097] Example 3: Zaleplon double-pulse release capsules independent of pH and preparation method thereof
[0098] The Zaleplon double-pulse release capsule of the present invention comprises an immediate-release tablet and a timed-release tablet, and the weight ratio of the active ingredient zaleplon contained in the immediate-release tablet and the timed-release tablet is 1:1,
[0099] The preparation method of zaleplon double-pulse release capsules, the preparation method first adopts the tablet press to compress the immediate-release tablet according to the commonly used techniques and methods in pharmacy; according to the formula, the tablet press is used to compress the drug-containing tablet core, and the drug-containing tablet core Use a coating pan to coat, and the coating film is attached to the drug-containing tablet core to obtain a time-release tablet; put the above-prepared immediate-release tablet and time-release tablet into a capsule to obtain zaleplon double p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com