Functionalized ionic liquid capable of selectively separating amino acids, preparation method and application thereof
An ionic liquid and amino acid technology, applied in the field of organic synthesis and biological separation and purification, can solve the problems of limited improvement ability of separation and purification process, single type of ionic liquid, diversity of difficult-to-functionalize ionic liquids, etc. Highly economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
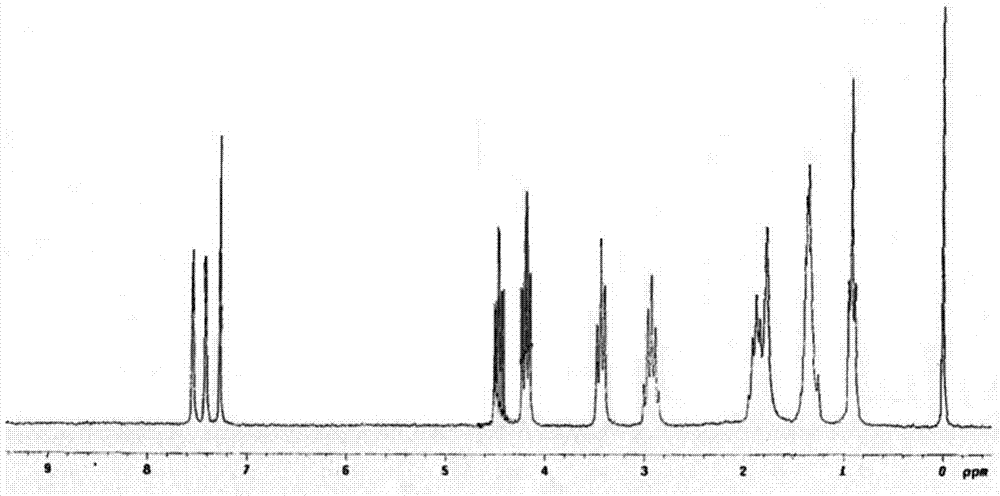
[0061] 1) Synthesis of 5,6,7,8-tetrahydropyridine [1,2-α]-3-(acetyl-n-butylamine) imidazole trifluorosulfonic acid isophenylalanine salt, ([4C-imCH 2 CONHBu][Tf-Phe]):
[0062] First, the anion (N-Trifluoromethanesulfonylphenylalanine, [Tf-Phe]) part was synthesized: at 0°C, thionyl chloride (0.24mol, 28.55g) was slowly added dropwise into a solution of phenylalanine (0.2mol, 33.04g). In methanol (100mL), after the dropwise addition was completed, the temperature was raised to reflux for 5h. After the reaction, filter, collect the filtrate, and remove the solvent under reduced pressure to obtain a white solid (yield94%). The intermediate (0.1mol, 18.00g) was dissolved in 100mL of dichloromethane, and placed at -78°C under nitrogen protection, and trifluoromethanesulfonic anhydride (0.1mol, 16.90mL) (CAS : 358-23-6) was slowly added dropwise into the system, after the dropwise addition was completed, it was raised to room temperature and stirred overnight. After the reacti...
Embodiment 2
[0074] 1) Synthesis of 6,7-dihydro-5H-pyrrole[1,2α]-3-(acetyl-alkylamine) imidazole trifluorosulfonic acid valine salt ([3C-imCH 2 CONHBu][Tf-Val]):
[0075] First, the anion (N-Trifluoromethanesulfonylvaline, [Tf-Val]) part was synthesized: at 0°C, thionyl chloride (0.24mol, 28.55g) was slowly added dropwise into methanol dissolved in valine (0.2mol, 23.43g) (100mL), after the dropwise addition was completed, the temperature was raised to reflux for 5h. After the reaction, filter, collect the filtrate, and remove the solvent under reduced pressure to obtain a white solid intermediate product (yield95%). The intermediate (0.15mol, 19.68g) was dissolved in 100mL of dichloromethane, and placed at -78°C under nitrogen protection, trifluoromethanesulfonic anhydride (0.2mol, 33.80mL) (CAS : 358-23-6) was slowly added dropwise into the system, and after the dropwise addition was completed, return to room temperature and stirred overnight. After the reaction, wash with dilute hy...
Embodiment 3
[0079] 1) Synthesis of 6,7-dihydro-5H-pyrrole [1,2-α]-3-(acetyl-alkylamine) imidazole trifluorosulfonic acid leucine salt, ([3C-imCH 2 CONHBu][Tf-Leu]):
[0080] First, the anion (N-Trifluoromethanesulfonylleucine, [Tf-Leu]) part was synthesized: at 0°C, thionyl chloride (0.24mol, 28.55g) was slowly added dropwise into methanol dissolved with leucine (0.2mol, 26.23g) (100mL), after the dropwise addition was completed, the temperature was raised to reflux for 5h. After the reaction, filter, collect the filtrate, and remove the solvent under reduced pressure to obtain a white solid intermediate product (yield92%). The intermediate (0.15mol, 21.78g) was dissolved in 100mL of dichloromethane, and placed at -78°C under nitrogen protection, trifluoromethanesulfonic anhydride (0.2mol, 33.80mL) (CAS : 358-23-6) was slowly added dropwise into the system, and after the dropwise addition was completed, return to room temperature and stirred overnight. After the reaction, wash with d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com