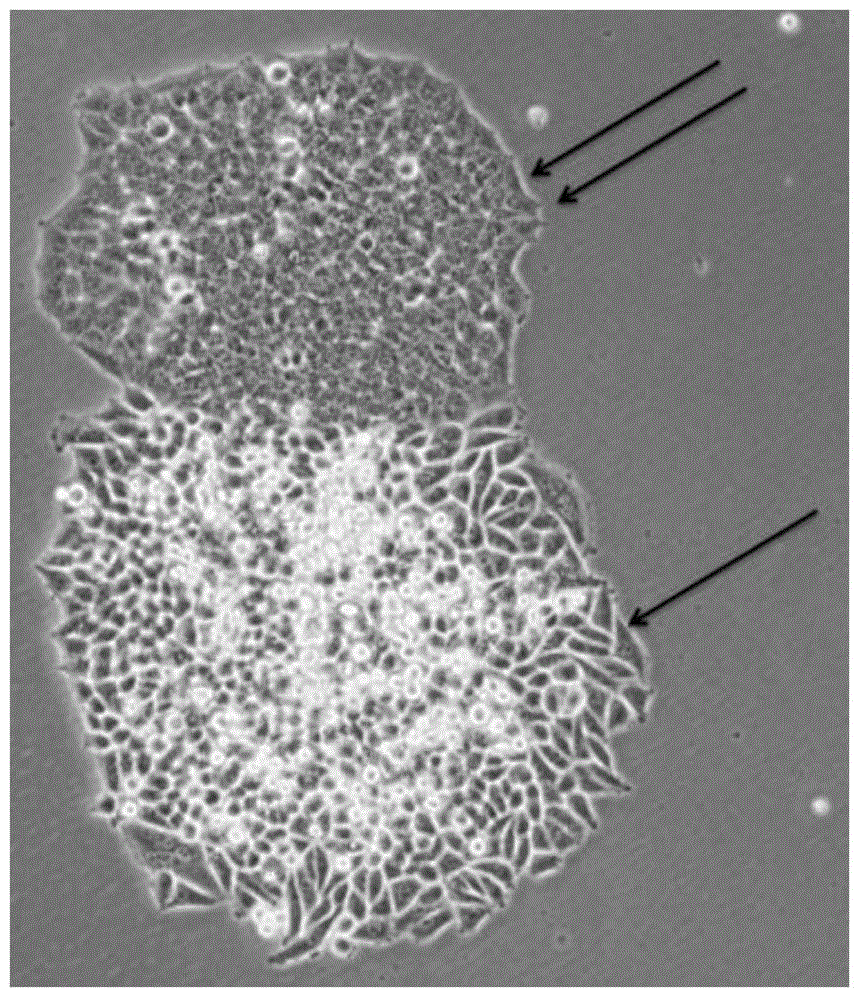
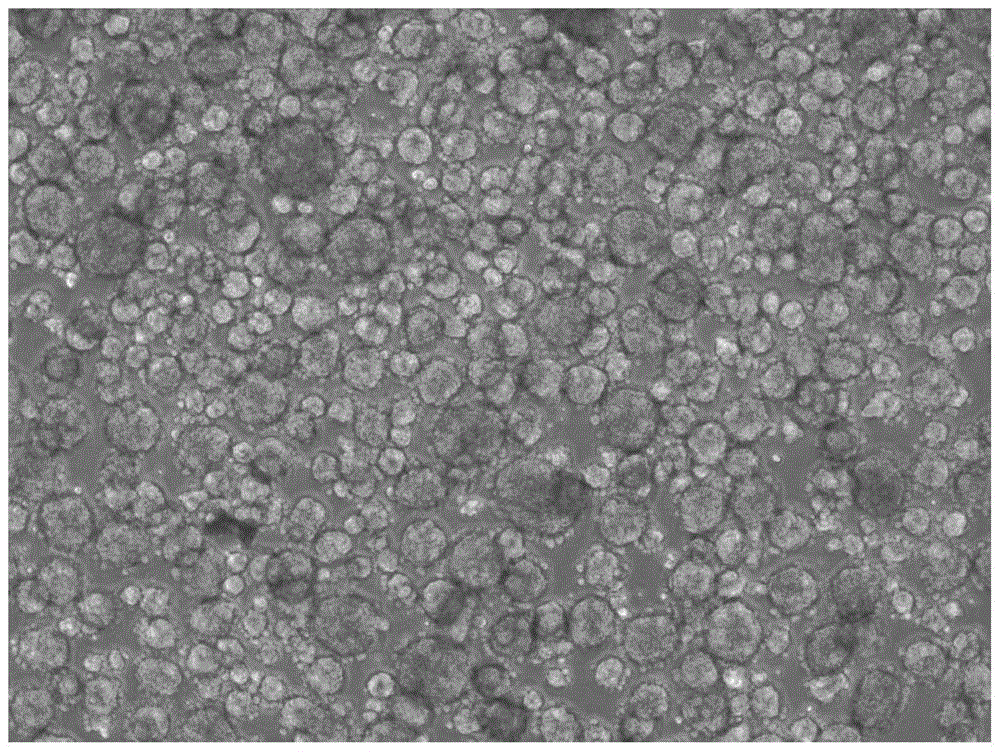
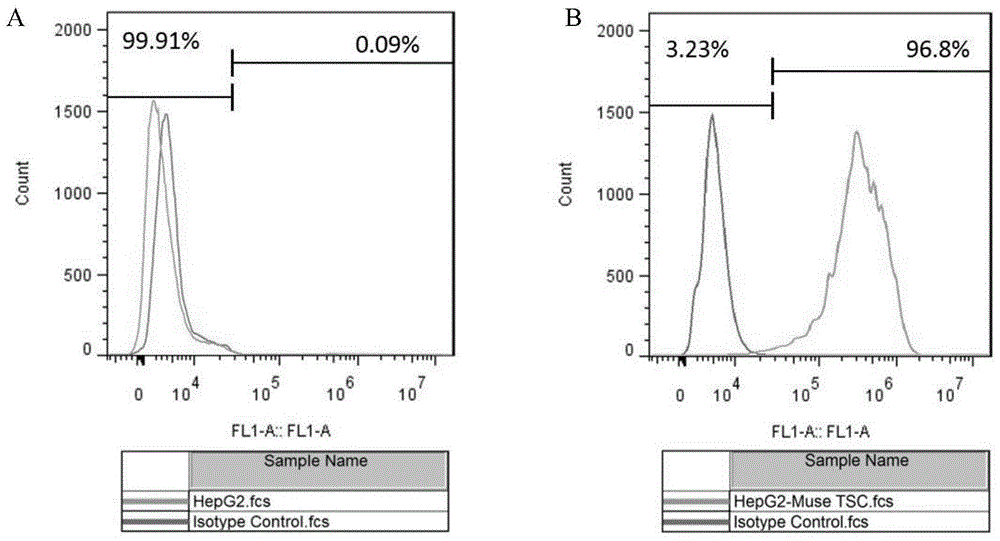
A method for isolating tumor stem cells
A technology of tumor stem cells and tumor cells, which is applied in the direction of tumor/cancer cells, animal cells, vertebrate cells, etc., can solve the problems of not determining TSC-specific surface markers, difficult to effectively separate TSCs, and no unified screening of surface markers, etc., to achieve The effect of good homogeneity, low cost and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Isolation method of HepG2 liver tumor stem cells
[0083] 1) Digest HepG2 adherent hepatoma cells with 0.25% trypsin solution at 37°C for 2-5 minutes to single cells, terminate with medium containing serum, centrifuge at 200g / min for 5min, and collect the precipitated cells; (here Steps can use other digestive enzymes instead of trypsin solution, such as TrypLE, Accutase, etc., the digestion time, digestion temperature, and centrifugation time are related to different types of tumor cells);
[0084] 2) Add 0.1% type I collagenase solution (diluted with basal medium DMEM / F12) to the collected cells above, every 1×10 6 Add 1mL enzyme solution to each cell and incubate at 37°C for 6h to simulate the digestive enzyme stress state; (in this step, other digestive enzymes can be used instead of 0.1% type I collagenase solution, such as 0.02-0.3% (w / v) type IV Collagenase solution, 0.1~3U / ml Dispase solution, 0.01~0.3% (w / v) trypsin solution, 0.01~1% (w / v) TrypLE enz...
Embodiment 2
[0105] Example 2 Isolation method of Hep3B liver tumor stem cells
[0106] 1) Use 0.25% trypsin solution to digest Hep3B adherent hepatoma cells at 37°C for 2-5 minutes to form a single cell, stop with medium containing serum, centrifuge at 200g / min for 5min, and collect the precipitated cells;
[0107] 2) Add 0.1% type I collagenase solution (diluted with basal medium DMEM / F12) to the collected cells above, every 1×10 6 Add 1mL enzyme solution to each cell and incubate at 37°C for 6h to simulate the stress state of digestive enzymes;
[0108] 3) Move the above-incubated cells to 4°C and incubate for 12-16 hours to simulate low temperature and digestive enzyme stress state;
[0109] 4) Wash the incubated cells with PBS buffer, mix well, centrifuge at 200g / min for 5min, discard the supernatant, wash away the enzyme solution, add DMEM / F12 culture medium to the cells precipitated in the centrifuge tube until the centrifuge tube is full , cover the cap of the centrifuge tube, se...
Embodiment 3
[0117] Example 3 Isolation method of glial tumor stem cells
[0118] 1) Digest adherent glioma cells U251 with 0.25% trypsin solution at 37°C for 4 minutes to single cells, terminate with medium containing serum, centrifuge at 200g / min for 5min, and collect the precipitated cells;
[0119] 2) Add 0.1% type I collagenase solution (prepared from DMEM culture medium) to the collected cells above, every 1×10 6 Add 1mL enzyme solution to each cell and incubate at 37°C for 6h to simulate the stress state of digestive enzymes;
[0120] 3) Move the above-incubated cells to 4°C and incubate for 12-16 hours to simulate low temperature and digestive enzyme stress state;
[0121] 4) Wash the incubated cells with PBS buffer, mix well, centrifuge at 200g / min for 5min, discard the supernatant, wash off the enzyme solution, add DMEM culture medium to the precipitated cells in the centrifuge tube until the centrifuge tube is full, cover Cap the centrifuge tube, seal the bottle with a parafil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com