Mouse anti-human PRRT2 monoclonal antibody, and preparation method and application thereof
A monoclonal antibody and antibody technology, applied in the biological field, can solve the problems of human PRRT2 expression profile research and functional research limitations, poor specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Design method of human PRRT2 immune antigen tandem polypeptide
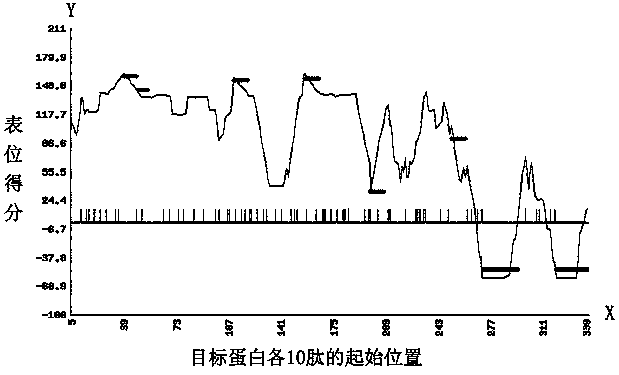
[0070] The antigen epitope design of the present invention adopts the Antigen Auto Designer antigen design program of Abmart Company. Surface peptides are determined by calculating parameters such as solvent accessibility, disorder index, domain prediction of protein-protein interactions, or any combination of the above. Six peptides with a length of 10 amino acids, high hydrophilicity, high antigenicity, non-signal peptide, non-transmembrane region and disordered region were selected as antigenic epitopes (attached figure 1 ). In order to avoid the generation of new antigenic epitopes between different peptide fragments, a weak immunogenic linker GGGGS was inserted between different peptide fragments to connect the peptide fragments in series. Finally, the tandem polypeptide sequence of PRRT2 protein was determined as: PPQVLAGVPD-GGGGS-PKPALQPELP-GGGGS-QETVSKPE VS-GGG GS-PSSQLAGPGV-EGPAPEPHS...
Embodiment 2
[0073] Example 2: Antigen expression and purification
[0074] The antigen sequence synthesized by the whole gene was digested with BamHI / EcoRI, then digested with BamHI / EcoRI and the recovered PET32a vector was ligated by T4 ligase at room temperature for 2 hours. The ligation product was incubated with Rosetta competent cells (Novagen, Merck, Germany) prepared by the CaCl2 method on ice (4°C) for 0.5h, and then heat-activated at 42°C for 90s, and 500ul of non-resistant LB was added to the bacterial solution after the heat shock After the liquid medium, slowly shake at 250rpm on a shaker at 37°C for 45min, and finally plate on LB plates containing corresponding antibiotics. Bacterial plates were grown overnight (16h) in a 37°C incubator.
[0075] The next day, the transformed colony was picked and placed in the self-inducing system medium, and expressed overnight at 37°C and 250rpm. The next day, the bacterial solution was centrifuged (6000g, 5min), the supernatant was disc...
Embodiment 3
[0077] Example 3: Preparation of Monoclonal Antibody
[0078] A. Animal immunity
[0079] Oligonucleotide adjuvant: 50ul aluminum adjuvant (Thermo Fisher, USA) + 1 μg DNA adjuvant, the sequence is tccatgacgttcctgacgtT, the bases in lowercase are the sites that need sulfo-modification, commissioned by Cyparkson (Shanghai, China) to synthesize .
[0080] Immunization method: 3 Balb / c mice, 8-10 weeks old, weighing 18-20g. 20 μg of antigen was fully emulsified with Fluorine’s complete adjuvant (Sigma), and the immunization sites were the hind paws, the root of the tail, the underarms of the forelimbs and the groin, about 50 μL per point. 20μg antigen was completely mixed with oligonucleotide adjuvant, and immunized mouse hind leg muscle, 50-100μL per spot.
[0081] On the 8th day, 10 μg of antigen was emulsified completely with Fluorine’s complete adjuvant (Sigma), and immunized the forelimb armpit and groin of mice, 50-100 μL per point. Mix 20μg of antigen with oligonucleoti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com