Tenofovir disoproxil fumarate tablet and preparation method thereof
A technology for tenofovir fumarate and pyrifurate tablets, applied in the field of tenofovir disoproxil fumarate composition and its preparation, to achieve good controllability, good fluidity and stable products good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
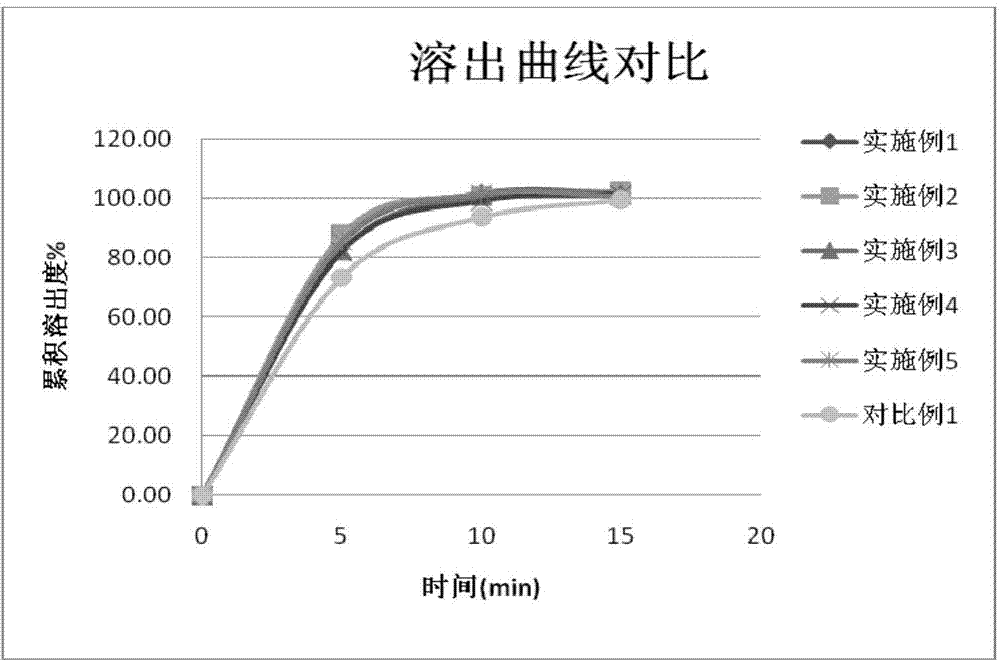
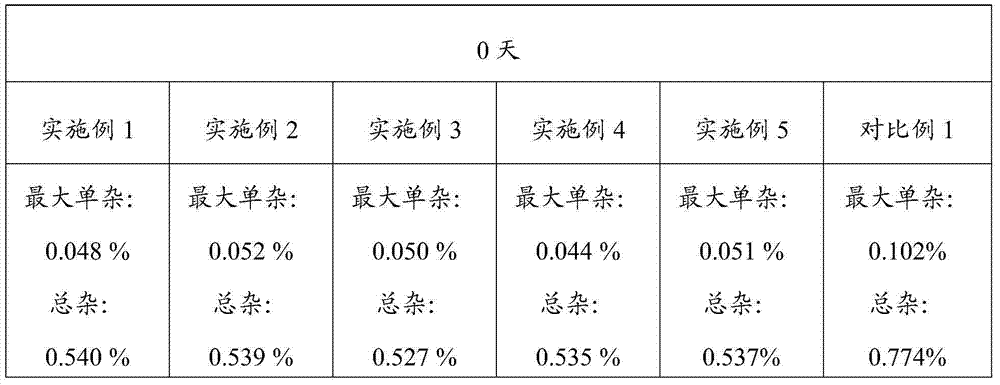
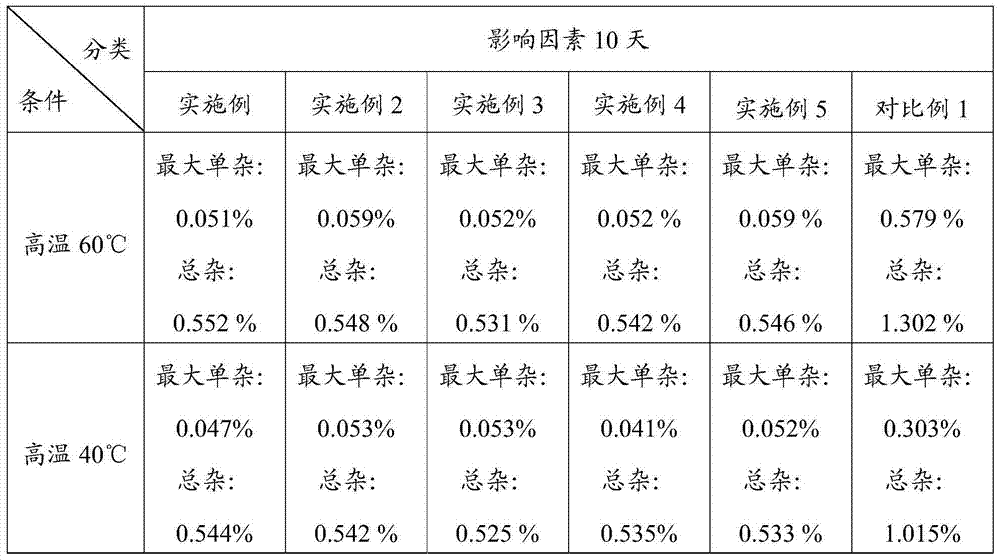
Image
Examples
Embodiment 1
[0042] Tenofovir Disoproxil Fumarate Tablets
[0043] Component
%(w / w)
tenofovir disoproxil fumarate
30
30
30
5
Crospovidone
4.6
0.2
0.2
[0044] 1. Preparation process:
[0045] 1.1 Preparation before ingredients
[0046] 1.1.1 Drying
[0047] The microcrystalline cellulose and the sodium carboxymethyl starch are dried at 105 DEG C and the water content is controlled between 0.5% and 2.0%.
[0048] 1.1.2 Screening
[0049] Take the pulverized tenofovir disoproxil fumarate and pass through an 80-mesh sieve for subsequent use.
[0050] 1.2 Ingredients
[0051] Picking and nuclear materials are carried out according to the batch feeding quantity.
[0052] 1.3 Hybrid
[0053] Weigh the prescribed amount of tenofovir disoproxil fumarate, microcrystalline cellulose, anhydrous la...
Embodiment 2
[0062] Tenofovir Disoproxil Fumarate Tablets
[0063] Component
%(w / w)
tenofovir disoproxil fumarate
35
25
[0064] anhydrous lactose
30
6
Crospovidone
3
Talc powder
0.5
0.5
[0065] 1. Preparation process:
[0066] 1.1 Preparation before ingredients
[0067] 1.1.1 Drying
[0068] The microcrystalline cellulose and the sodium carboxymethyl starch are dried at 105 DEG C and the water content is controlled between 0.5% and 2.0%.
[0069] 1.1.2 Screening
[0070] Take the pulverized tenofovir disoproxil fumarate and pass through a 60-mesh sieve for subsequent use.
[0071] 1.2 Ingredients
[0072] Picking and nuclear materials are carried out according to the batch feeding quantity.
[0073] 1.3 Hybrid
[0074] Weigh the prescribed amount of tenofovir disoproxil fumarate, microcrystalline cellulo...
Embodiment 3
[0083] Tenofovir Disoproxil Fumarate Tablets
[0084] Component
%(w / w)
tenofovir disoproxil fumarate
40
microcrystalline cellulose
10
anhydrous lactose
30
15
Crospovidone
3
Talc powder
0.5
colloidal silica
1.5
[0085] 1. Preparation process:
[0086] 1.1 Preparation before ingredients
[0087] 1.1.1 Drying
[0088] The microcrystalline cellulose and the sodium carboxymethyl starch are dried at 105 DEG C and the water content is controlled between 0.5% and 2.0%.
[0089] 1.1.2 Screening
[0090] Take the pulverized tenofovir disoproxil fumarate and pass through a 40-mesh sieve for subsequent use.
[0091] 1.2 Ingredients
[0092] Picking and nuclear materials are carried out according to the batch feeding quantity.
[0093] 1.3 Hybrid
[0094] Weigh the prescribed amount of tenofovir disoproxil fumarate, microcrystalline cellulose, anhydrous lact...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com