Recombinant expression method of human lysozyme
A technology of human lysozyme and expression cassette, applied in the field of biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, the vector construction that contains human lysozyme coding sequence
[0057] The nucleotide sequence of the natural human lysozyme cDNA is as SEQ ID NO: 1 (the 1-54th position at the 5' end encodes a signal peptide), which encodes the amino acid sequence shown in SEQ ID NO: 16.
[0058] In order to utilize the expression of human lysozyme, the sequence was designed and modified, and the 1-54th signal peptide coding sequence was removed at the 5' end of human lysozyme cDNA, and the coding sequence CTC GAG AAG AGA with part of the 3' end of the yeast α signal peptide was added (SEQ ID NO:8), a synthetic sequence such as SEQ ID NO:2, at the 3' end of the DNA is the translation termination signal TAA, the DNA is inserted into the PUC18 vector (purchased from New England Biolabs), named as PUC18- HLY.
Embodiment 2
[0059] Embodiment 2, human lysozyme cDNA sequence optimization
[0060] Considering the codons preferred by Pichia pastoris, the ratio design of AT:GC, the optimization of mRNA secondary structure, the size and distribution of the base AT-rich region, the GC cluster (GC cluster) or the G cluster (G cluster) On the basis of a series of factors such as distribution, the natural lysozyme cDNA sequence (SEQ ID NO: 1) is optimized, and the sequence GAATTC of the EcoRI restriction site is added to the 3' end, and the optimized lysozyme matures The peptide cDNA sequence is SEQ ID NO:3.
Embodiment 3
[0061] Example 3. Construction of phase bean lectin signal peptide-optimized lysozyme cDNA sequence
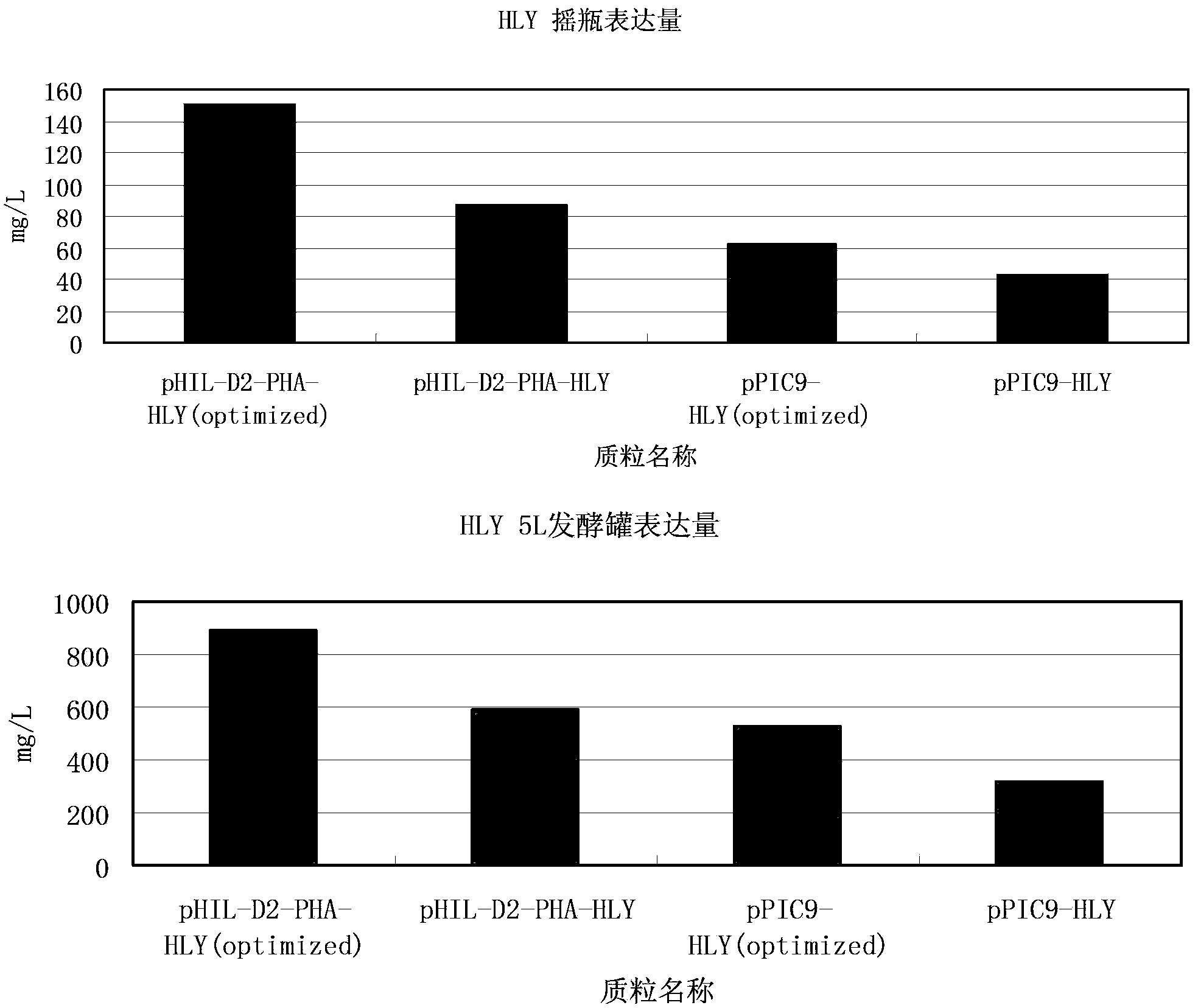
[0062] Phaseolin signal peptide (PHA) sequence has a nucleotide sequence such as SEQ ID NO:4, and an amino acid sequence such as SEQ ID NO:15. Artificially synthesize the PHA-HLY (optimized) DNA sequence, add the PHA signal peptide coding sequence and the EcoRI restriction site at the 5' end to obtain the PHA-HLY sequence, and its sequence is the EcoRI restriction site sequence followed by the PHA signal peptide The sequence (SEQ ID NO:4) is followed by the sequence-optimized mature peptide sequence of lysozyme, followed by the terminator sequence, and finally the EcoRI restriction site sequence. The artificially synthesized full sequence is SEQ ID NO:5. The synthetic sequence was inserted into the PUC18 vector to construct a PUC18-PHA-HLY (optimized) vector.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com