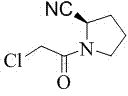
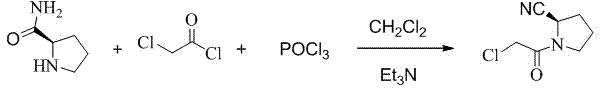Preparation method of (S)-1-(2-chloracetyl)pyrrolidine-2-carbonitrile
A technology of chloroacetyl and pyrrolidine, which is applied in the field of preparation of 1-pyrrolidine-2-carbonitrile, can solve the problems of difficult purification, high equipment requirements, and low yield, and achieve the protection of green resources, low raw material cost, Good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile:
[0017] Add 25g of L-prolinamide, 250ml of dichloromethane, and 25g of triethylamine into a 1000ml dry reaction flask to form a mixed solution. Cool the mixed solution to -20°C to -25°C, and dropwise add 26g of The mixture prepared by chloroacetyl chloride and 50ml of dichloromethane was added dropwise and kept at -20°C for 3 hours with stirring and reaction to obtain reaction solution 1 containing 1-chloroacetylpyrrolidine-2-carboxamide.
[0018] Raise the temperature of the above reaction solution 1 to 5°C, control the temperature of the reaction solution 1 to 5°C-15°C, add 45g of phosphorus oxychloride dropwise, after the dropwise addition, keep the reaction at 5°C-15°C for 1 hour to obtain the reaction solution two. Slowly add 100ml of water to the reaction solution 2, the internal temperature does not exceed 20°C, stir for 30 minutes, let stand to separate layers, extract the water layer with dichlo...
Embodiment 2
[0020] Preparation of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile:
[0021] Add 25g of L-prolinamide, 250ml of dichloromethane, and 35g of diisopropylethylamine into a 1000ml dry reaction flask to form a mixed solution, cool the mixed solution to -45°C to -50°C, and drop it into the reaction flask Add the mixed solution prepared by 30g of chloroacetyl chloride and 50ml of dichloromethane. After the dropwise addition, keep stirring at -50°C for 0.5 hours to obtain a reaction solution containing 1-chloroacetylpyrrolidine-2-carboxamide. .
[0022] Raise the temperature of the above reaction solution 1 to -5°C, control the temperature of the reaction solution 1 at -15°C to 5°C, add 40 g of phosphorus oxychloride dropwise, after the dropwise addition, keep the reaction at -5°C to 5°C for 0.5 hours, Obtain reaction solution 2. Slowly add 110ml of water to the reaction solution 2, the internal temperature does not exceed 20°C, stir for 30 minutes, let stand to separate layers,...
Embodiment 3
[0024] Preparation of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile:
[0025] Add 25g of L-prolinamide, 250ml of dichloromethane, and 31g of 4-dimethylaminopyridine into a 1000ml dry reaction flask to form a mixed solution. Cool the mixed solution to -30°C to -35°C, and dropwise add The mixture prepared from 30g of chloroacetyl chloride and 50ml of dichloromethane was added dropwise and kept at -30°C for 4 hours with stirring and reaction to obtain a reaction solution 1 containing 1-chloroacetylpyrrolidine-2-carboxamide.
[0026] The temperature of the above reaction solution 1 was raised to -5°C, the temperature of the reaction solution 1 was controlled at -5°C to 5°C, and 35 g of phosphorus oxychloride was added dropwise. Obtain reaction solution 2. Slowly add 120ml of water to the reaction solution 2, the internal temperature does not exceed 20°C, stir for 30 minutes, let stand to separate layers, extract the water layer with dichloromethane twice, each time the amount ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com