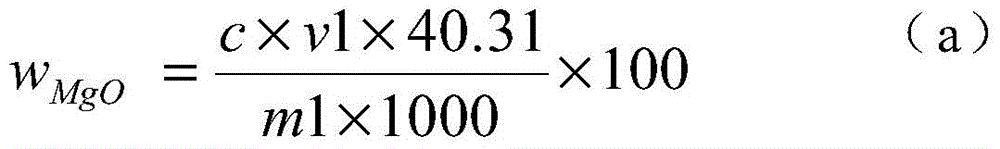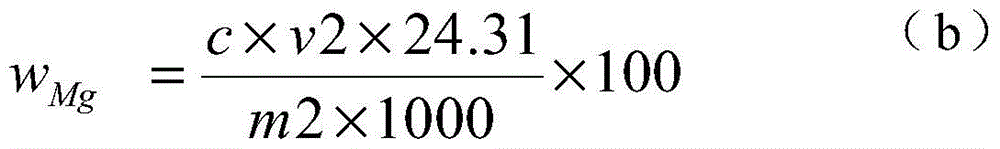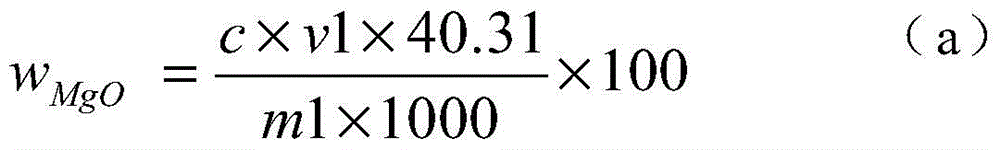A kind of passive magnesium chemical phase analysis method
A technology of chemical substance and phase analysis, which is applied in the direction of analyzing materials through chemical reactions, material analysis through observation of the influence of chemical indicators, preparation of test samples, etc., which can solve the problems of cumbersome operation and long time consumption , to achieve the effect of simple operation, fast method and good practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Weigh 0.1000g of the sample into a 250ml Erlenmeyer flask, add 4g of iodine, 50ml of methanol, heat in a water bath (50-60°C), electromagnetically stir, and reflux for 40 minutes to obtain a sample solution.
[0025] 2) Filter the sample solution with a slow filter paper, rinse the precipitate on the slow filter paper with methanol until the precipitate is colorless, and collect the filtrate in a 250 ml beaker.
[0026] 3) Put the precipitate together with the filter paper into a 250ml Erlenmeyer flask, add 10ml of concentrated nitric acid and 5ml of concentrated perchloric acid, heat and nitrify in a medium temperature furnace, and blow perchloric acid fumes until nearly dry.
[0027] 4) Cool the precipitate that the concentrated perchloric acid has smoked, add 5 milliliters of concentrated hydrochloric acid to dissolve the salts, transfer to a 100 milliliter volumetric flask, and dilute to the mark with water to obtain a magnesium oxide test solution.
[0028] 5) P...
Embodiment 2
[0038] 1) Weigh 0.1500g of the sample into a 250ml Erlenmeyer flask, add 6g of iodine and 80ml of methanol, heat in a water bath (50-60°C), electromagnetically stir, and reflux for 45 minutes to obtain a sample solution.
[0039] 2) Filter the sample solution with a slow filter paper, rinse the precipitate on the slow filter paper with methanol until the precipitate is colorless, and collect the filtrate in a 250 ml beaker.
[0040] 3) Put the precipitate together with the filter paper into a 250ml Erlenmeyer flask, add 20ml of concentrated nitric acid and 8ml of concentrated perchloric acid, heat and nitrify in a medium-temperature furnace, and emit perchloric acid fumes until nearly dry.
[0041] 4) Cool the precipitate that the concentrated perchloric acid has smoked, add 5 milliliters of concentrated hydrochloric acid to dissolve the salts, transfer to a 100 milliliter volumetric flask, and dilute to the mark with water to obtain a magnesium oxide test solution.
[0042] 5...
Embodiment 3
[0052] 1) Weigh 0.2000g of the sample into a 250ml Erlenmeyer flask, add 8g of iodine and 100ml of methanol, heat in a water bath (50-60°C), stir electromagnetically, and reflux for 50 minutes to obtain a sample solution.
[0053] 2) Filter the sample solution with a slow filter paper, rinse the precipitate on the slow filter paper with methanol until the precipitate is colorless, and collect the filtrate in a 250 ml beaker.
[0054] 3) Put the precipitate together with the filter paper into a 250ml Erlenmeyer flask, add 30ml of concentrated nitric acid and 10ml of concentrated perchloric acid, heat and nitrify in a medium-temperature furnace, and emit perchloric acid fumes until nearly dry.
[0055] 4) Cool the precipitate that the concentrated perchloric acid has smoked, add 5 milliliters of concentrated hydrochloric acid to dissolve the salts, transfer to a 100 milliliter volumetric flask, and dilute to the mark with water to obtain a magnesium oxide test solution.
[0056] 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com