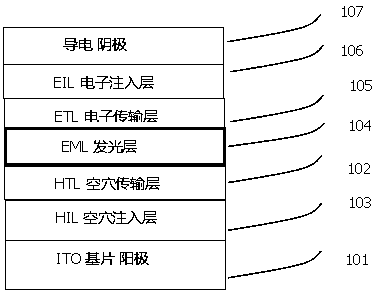
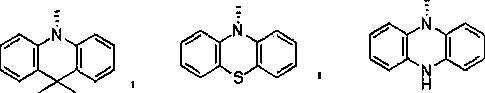
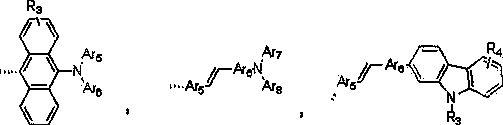
Blue light-emitting material
A technology of the same compound, applied in the field of blue light-emitting materials, can solve problems such as poor preparation or performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1. Synthesis of Compound 10:
[0120] .
[0121] Synthesis of intermediate 3,6-diboronate-5,8-diphenyltriphenylene: In a 100 mL three-necked flask with a thermometer and a condenser tube, add 2.15 g (4 mmol) of 3,6-dibromo-5,8-diphenyltriphenylene in sequence, pinacol diboronate 2.54g (10 mmol), 0.2g (0.2 mmol) of tribenzylideneacetone dipalladium, 0.25g (0.6mmol) of S-phos, 1.17g (12 mmol) of potassium acetate, 20ml of toluene, replaced with nitrogen, heated to reflux for reaction 16h. Cool to room temperature, filter, collect the filtrate, use a short column of silica gel, use pure n-hexane:dichloromethane=4:1 as the mobile phase, and receive 1.58g (65%) of white solid, MS=612.
[0122] Synthesis of the final product: In a 100 mL three-neck flask with a thermometer and a condenser tube, add 2.00 g (3.16 mmol) of 3,6-diboronate-5,8-diphenyltriphenylene in sequence, 9-Bromo-10-(2-naphthyl)anthracene 2.42g (6.32 mmol), Pd(PPh 3 ) 4 0.2g (0.16 mmol...
Embodiment 2
[0123] Example 2. Synthesis of Compound 21:
[0124] .
[0125] Synthesis of Intermediate M1: In a 250 mL three-necked flask with a thermometer and a condenser tube, add 6.94 g (18.96 mmol) of 3,6-dibromophenanthrenequinone, 4.18 g (19.9 mmol) of dibenzyl ketone, and 1.78 g (30.48 mmol) of KOH ), methanol 120ml, heated to reflux for 2h. The reaction was monitored by TLC plate until complete. Cool to room temperature, filter, wash the filter residue with methanol, and filter to obtain the desired product. 6.4 g (62.5%) of a brown solid is received; it is directly used in the next reaction without purification.
[0126] Synthesis of Intermediate M2: In a 100 mL three-necked flask with a thermometer and a condenser tube, 4.5 g (8.325 mmol) of M1, 1.8 g (18.3 mmol) of trimethylsilylacetylene, and 45 ml of o-xylene were sequentially added, and the temperature was raised to reflux for 12 hours. Cooled to room temperature, filtered, the filtrate was precipitated with metha...
Embodiment 3
[0130] Example 3. Synthesis of Compound 22:
[0131] .
[0132] Synthesis of intermediate M6: In a 100 mL three-necked flask with a thermometer and a condenser tube, add 14.8 g (109 mmol) of p-cymeniline, 19.2 g (72.87 mmol) of 4-bromodibenzothiophene, and 1 g of dipalladium ( 1.09 mmol), BINAP 1.35g (21.8mmol), sodium tert-butoxide 13.9g (145 mmol), toluene 180ml, nitrogen replacement, heated to reflux for 3h. Cool to room temperature, filter, collect the filtrate, use a short column of silica gel, use pure n-hexane: dichloromethane = 4:1 as the mobile phase, and receive 18.9 g (79%) of a white solid.
[0133] Synthesis of the final product: In a 100 mL three-necked flask with a thermometer and a condenser tube, add M6 0.66g (2.1mmol), M3 0.538g (1 mmol), tribenzylideneacetone dipalladium 0.048g (0.05 mmol), P(t- 0.16 g (0.2 mmol) of Bu)3, 0.38 g (4 mmol) of sodium tert-butoxide, 25 ml of toluene, replaced with nitrogen, and heated to reflux for 16 hours. Cool to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com