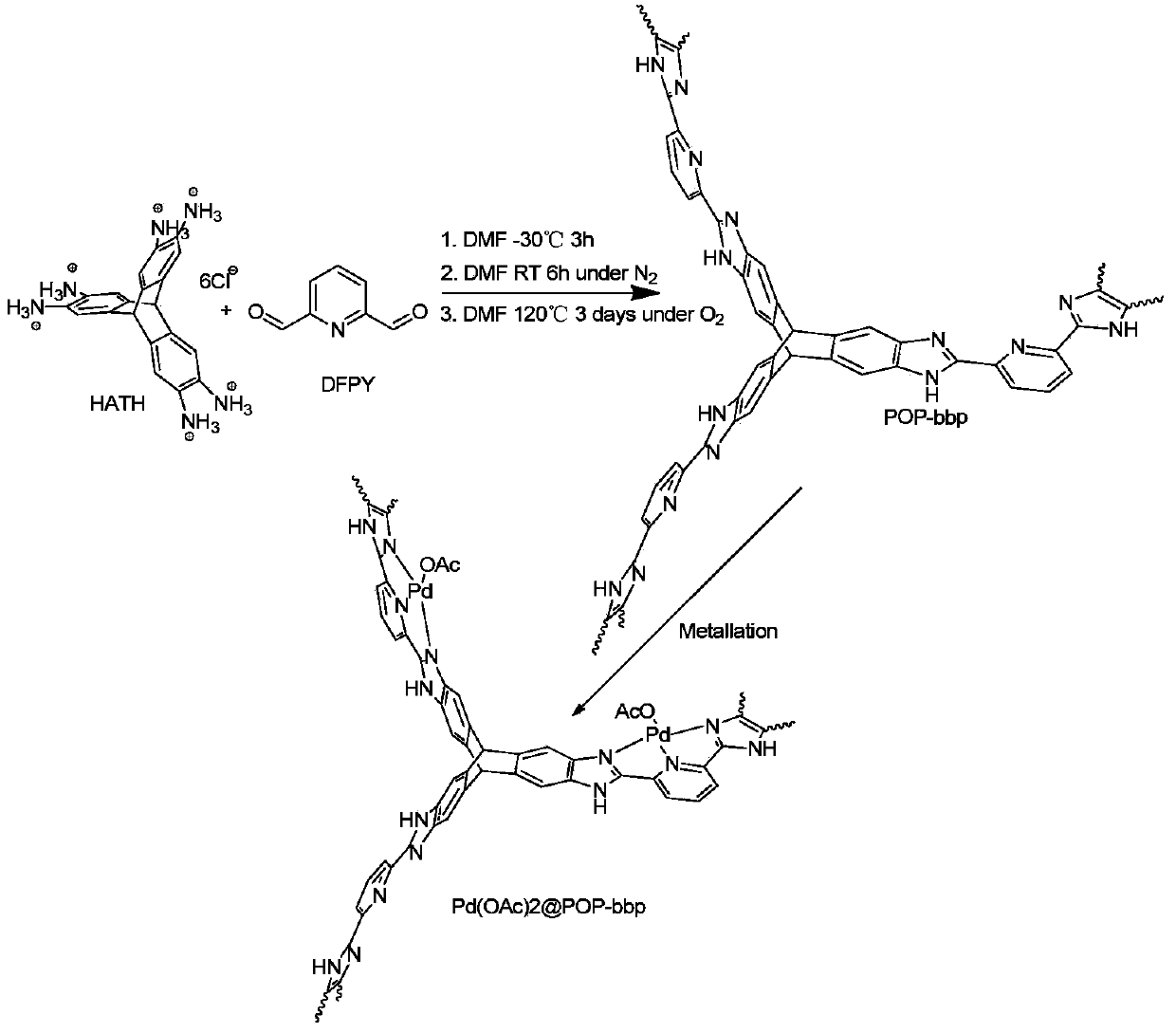
Nitrogen-containing tridentate ligand organic microporous polymer material as well as preparation and application thereof
A porous polymer and organic technology, applied in the preparation of organic compounds, carbon-based compounds, organic compound/hydride/coordination complex catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] A more preferred preparation method is as follows:
[0081] Step 1) In an inert solvent, add hexaaminotriptycene hydrochloride (HATH), and under the protection of nitrogen, lower the temperature of the reaction to below 0° C. and stir.
[0082] Step 2) Prepare an inert solvent solution of 2,6-diformylpyridine (DFPY), and remove oxygen by bubbling.
[0083] Step 3) Slowly add the solution prepared in step 2 dropwise to the reaction system in step 1. After the addition process is completed, keep the reaction temperature constant for several hours. Then the temperature of the reaction system was gradually raised to room temperature.
[0084] Step 4) Bubble air into the reaction system which was raised to room temperature. Then the reaction system was transferred to a sealed tube and sealed, and the temperature was gradually raised for reaction.
[0085] Step 5) The reaction system in step 4 was cooled to room temperature to obtain a yellow fluffy precipitate. After fil...
Embodiment 1
[0119] The synthesis of embodiment 1 organic microporous polymer
[0120] Reactions such as figure 1 As indicated, 2,3,6.7,14,15-hexaaminotriptycene hydrochloride (HATH) (90 mg, 0.16 mmol) and 30 mL DMF were added to a 100 mL round bottom flask. system in N 2 The mixture was stirred at -30°C for 10 min, and then a DMF solution of 2,6-diformylpyridine (DFPY) (32.4mg, 0.24mmol, 20mL DMF) was slowly added dropwise by bubbling deoxygenation. The reaction system was stirred at -30°C for 6 h and then gradually warmed to room temperature. A tan precipitate was observed to develop during stirring. Before heating, air was bubbled through the reaction system for 30 min and transferred to a 100 mL sealed tube. The sealed reaction system was heated at 140° C. for 72 hours to obtain a fluffy yellow precipitate. Filtration, the obtained yellow precipitate was successively washed with DMF, acetone, H 2 O, 0.5M HCl, 0.5M NaOH, H 2 O and acetone washes, followed by acetone / CH 2 Cl 2 (...
Embodiment 2
[0124] Embodiment 2 Catalyst loading process
[0125] Pd(OAc) was added to a 25mL round bottom flask 2 (40mg) and 10mL CH 2 Cl 2 , stirred until all the solids were dissolved, then added POP-bbp (110 mg). The reaction system was heated at 60°C for 24 hours, and the solid obtained by filtration was leached with acetone for 12 hours in a Soxhlet extractor. The supported catalyst Pd(OAc) was obtained by vacuum drying overnight at 100°C 2 POP-bbp (102 mg). Elemental analysis: theoretical value, C (47.7%), H (3.80%), N (12.46%); experimental value, C (48.58%), H (3.77%), N (13.60%). ICP-AES analysis: Pd content, 5.89 wt%.
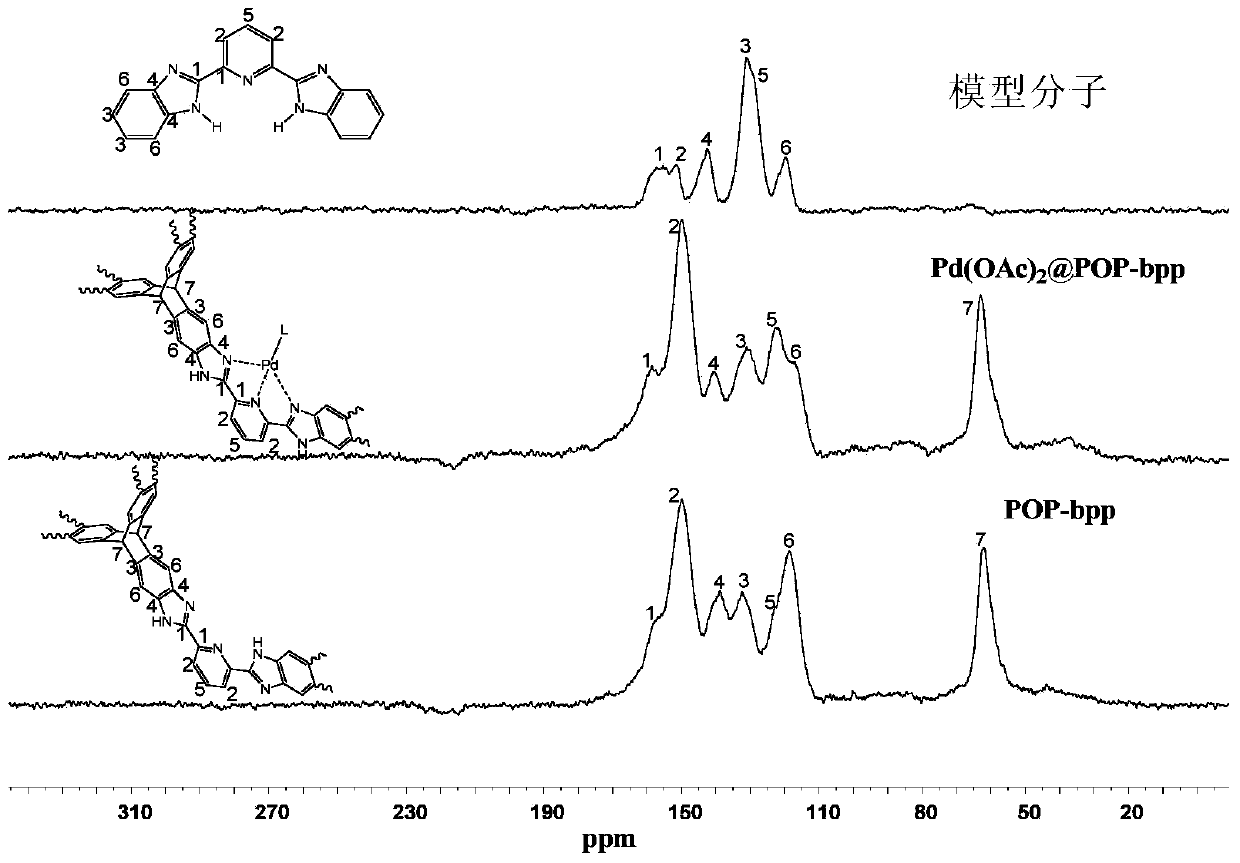
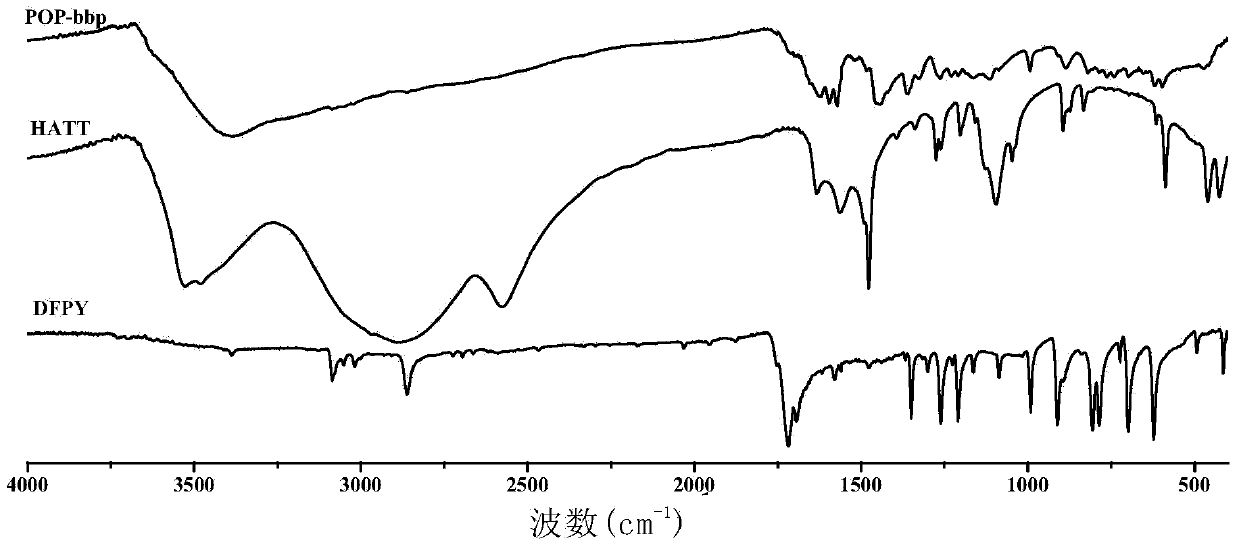
[0126] image 3 , Figure 4 , Figure 5 For POP-bbp and Pd(OAc) 2 The infrared spectrum comparison charts of POP-bbp and raw materials and model molecules show that there are 2,6-bis(2-benzimidazolyl)pyridine (bbp) basic units and triptycene skeletons in the product, indicating that the product contains The 2,6-bis(2-benzimidazolyl)pyridine (bbp) basi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com