Biomarkers for bladder cancer and methods using the same
A technology for biomarkers and bladder cancer, applied in biological testing, biomaterial analysis, compound screening, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1: Biomarkers for Bladder Cancer
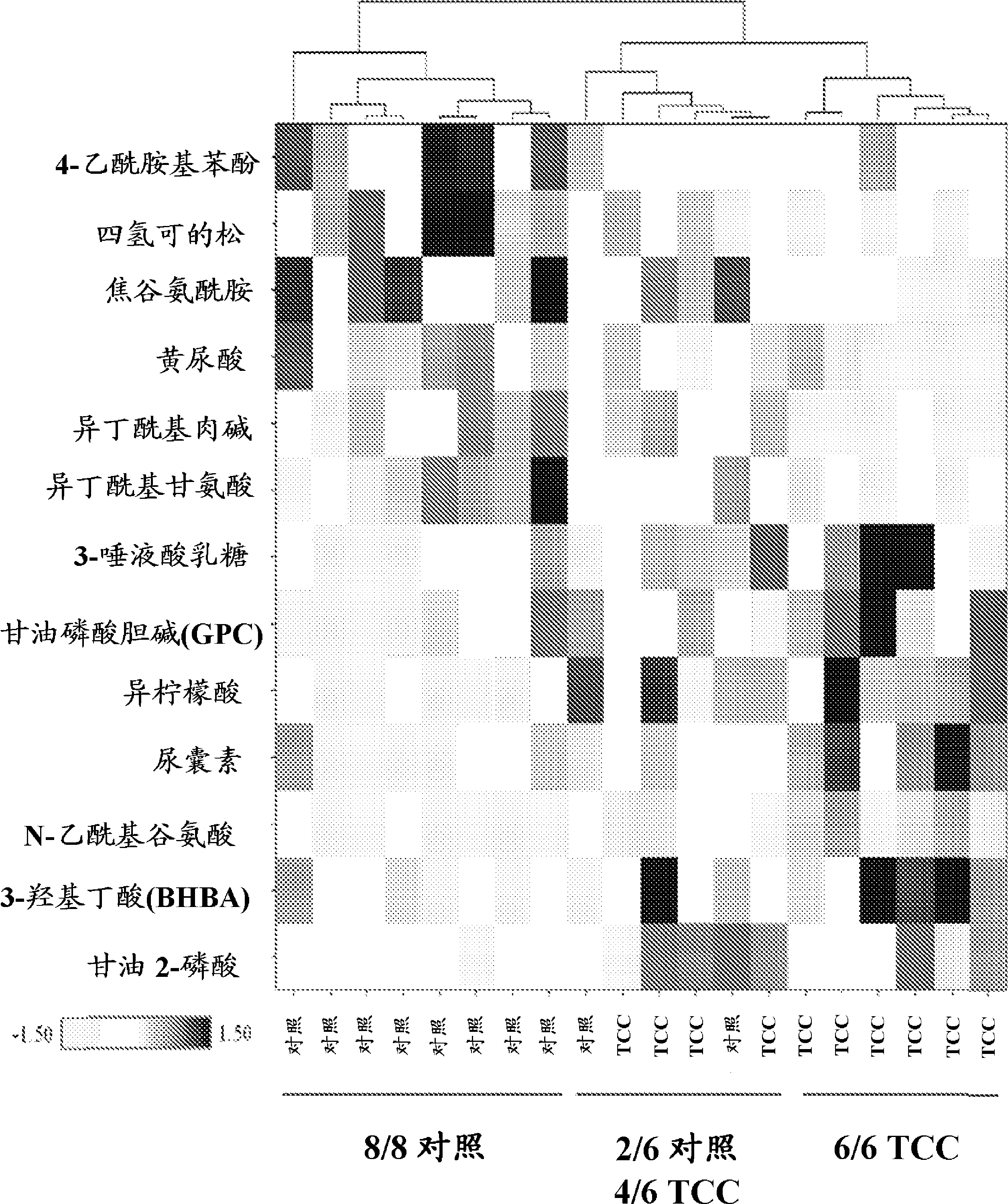
[0153] Biomarkers were discovered by (1) analyzing urine samples from different groups of human subjects to determine the levels of metabolites in the samples, and then (2) performing statistical analysis of the results to determine differences between the 2 groups metabolites in.
[0154] Two studies were conducted to identify biomarkers for bladder cancer. In Study 1, 10 control urine samples collected from subjects without bladder cancer and 10 urine samples from subjects with bladder cancer (urothelial transitional cell carcinoma) were analyzed . Age, race, and gender were all strictly controlled to minimize the effect of variables that confound demographic effects. All subjects were Caucasian males. The mean age of the bladder cancer cohort was 71.1, and the mean age of the control cohort was 67.7. For age, the p-value of the paired t-test analysis was 0.2, which indicated that age was not significantly different be...
Embodiment 2
[0163] Example 2. Classification of Subjects Based on Urine Biomarkers in a Statistical Model
[0164] a. BCA and non-cancer
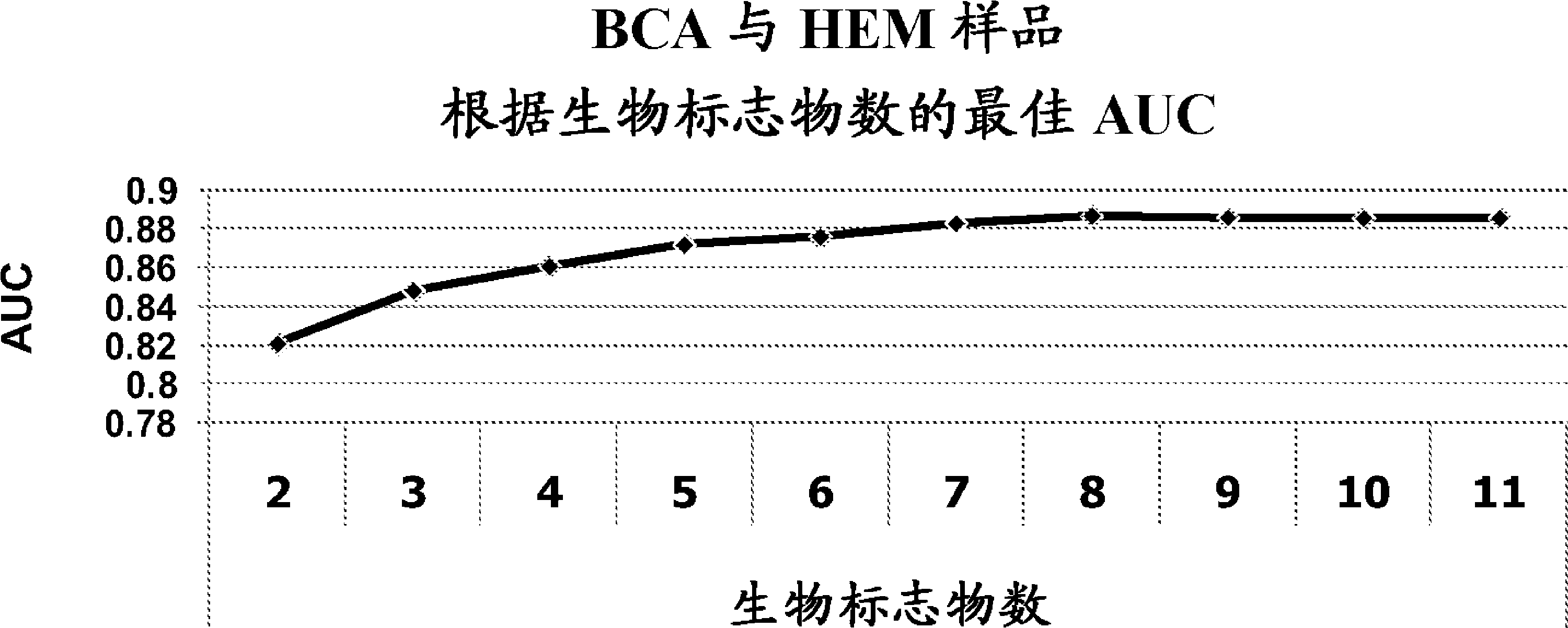
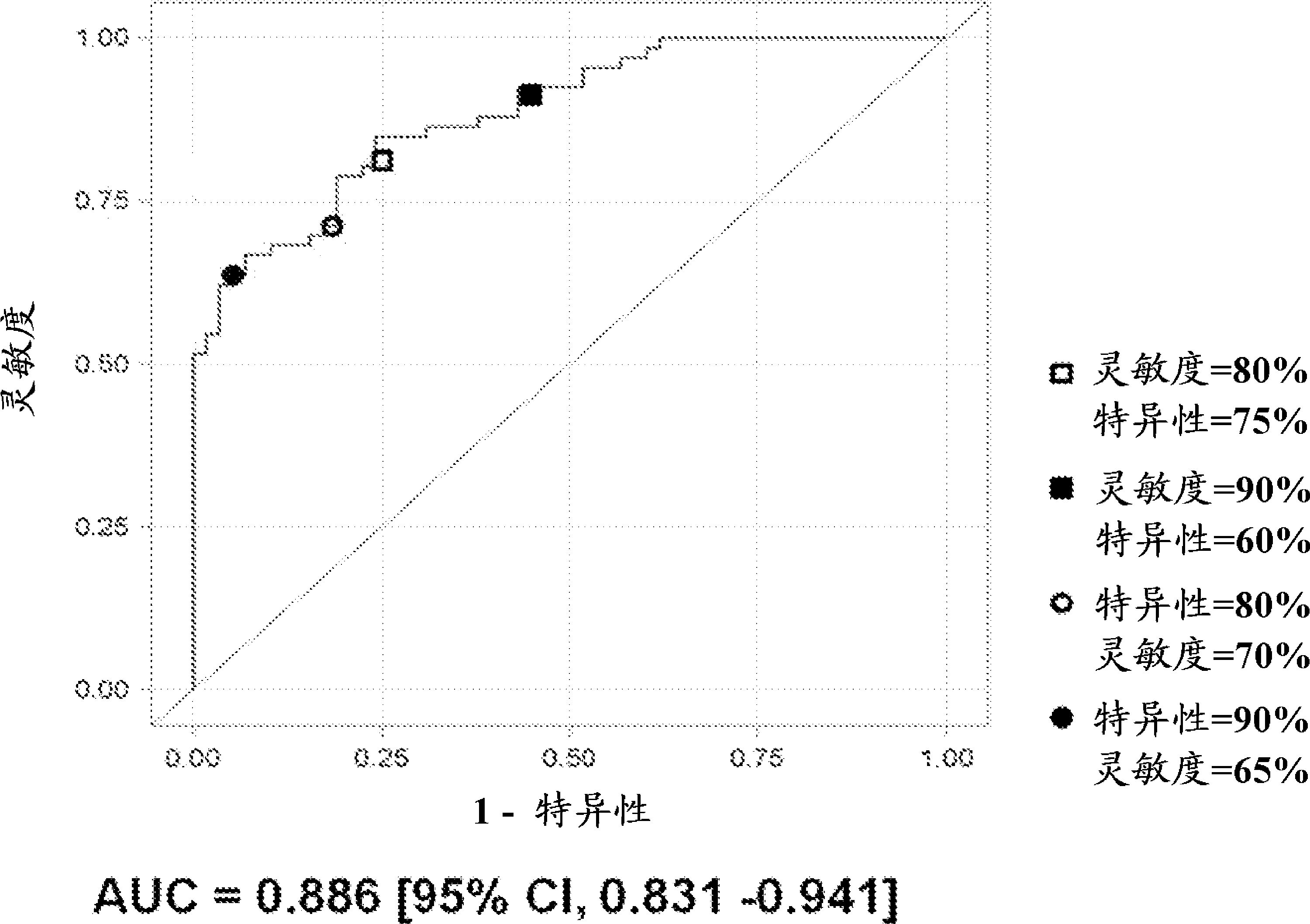
[0165] A number of analytical methods are available to evaluate the utility of identified biomarkers for diagnosing a patient condition (eg, whether a patient has bladder cancer). Two simple methods are used below: principal component analysis and hierarchical clustering using Pearson correlation.
[0166] In one method of analysis, principal component analysis was performed to model the classification of subjects as controls (non-cancer) or bladder cancer (TCC). The data used for the principal component analysis model were obtained from the osmolarity-normalized data of the urine samples from Study 1 of Example 1 (i.e., 10 control urine samples collected from subjects without bladder cancer and 10 urine samples from subjects with bladder cancer (urothelial transitional cell carcinoma).
[0167] Using the model derived by principal component ana...
Embodiment 3
[0190] Example 3. Biomarkers for bladder cancer staging
[0191] Bladder cancer staging provides an indication of how far the bladder tumor has spread. Tumor staging is used to select treatment options and estimate a patient's prognosis. Bladder tumor stages range from T0 (no evidence of a primary tumor, the earliest stage) to T4 (the tumor has spread beyond the fat tissue surrounding the bladder into nearby organs, the latest stage). Early stages of bladder cancer can also be characterized as carcinoma in situ (CIS), which means cells that proliferate abnormally but are still contained within the bladder.
[0192] To identify biomarkers of disease stage and / or progression, 21 low-stage BCA (CIS, T0, T1) subjects, 42 high-stage BCA (T2-T4) subjects, and 89 normal subjects were tested. Urine samples from the patients were subjected to metabolome analysis. After determining the levels of metabolites, the data were analyzed using one-way ANOVA comparatively to identify bioma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com