Tetra-substituted perylene diimide dimer, preparation method of tetra-substituted perylene diimide dimer and use of tetra-substituted perylene diimide dimer in organic photovoltaic device
A perylene diimide dimer and dimer technology, which is applied in the fields of electric solid-state devices, semiconductor devices, semiconductor/solid-state device manufacturing, etc., can solve the problems of limiting the application of photovoltaic materials, poor solubility and film-forming properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1. Preparation of asymmetrically substituted 1,12-dialkoxy-6-bromo-perylenediimide represented by formula II (compound 3a)
[0100]
[0101] The preparation of compound 1 in this note was synthesized according to the literature (Y.G.Zhen, H.L.Qian, J.F.Xiang, J.Q.Qu, Z.H.Wang, Org.Let,., 2009, 11, 3084-3087).
[0102] Dissolve 1 (136.4 mg, 0.2 mmol) in 10 mL of DMF, add methoxyethanol (76.1 mg, 1.0 mmol) and K 2 CO 3 (140.0mg, 1.0mmol), under the heating condition of 80 ℃, stir reaction for 24 hours. After the reaction was completed, the reaction solution was poured into 50 mL of water, the precipitate was collected by filtration, dissolved in a mixed solvent of 50 mL of dichloromethane and 50 mL of water, the dichloromethane layer was collected by liquid separation, and the dichloromethane was distilled off under reduced pressure to obtain purple A black solid was passed through a column with H60 silica gel to obtain product 2a (83.9 mg, 0.110 mmol, yield:...
Embodiment 2
[0107] Example 2. Preparation of asymmetrically substituted 1,12-dialkoxy-6-bromo-perylenediimide represented by formula II (compound 3b)
[0108]
[0109] Dissolve 1 (136.4 mg, 0.2 mmol) in 10 mL of DMF, add methoxyethanol (74.1 mg, 1.0 mmol) and K 2 CO 3 (140.0mg, 1.0mmol), under the heating condition of 80 ℃, stir reaction for 24 hours. After the reaction was completed, the reaction solution was poured into 50 mL of water, the precipitate was collected by filtration, dissolved in a mixed solvent of 50 mL of dichloromethane and 50 mL of water, the dichloromethane layer was collected by liquid separation, and the dichloromethane was distilled off under reduced pressure to obtain purple A black solid was passed through a column with H60 silica gel to obtain product 2b (86.6 mg, 0.114 mmol, yield: 57%).
[0110] Dissolve 2b (75.8 mg, 0.1 mmol) in 20 mL of dichloromethane, add 1 mL of elemental bromine dropwise under heating and reflux, and continue heating to reflux overni...
Embodiment 3
[0114] Example 3, Preparation of asymmetrically substituted perylene diimide dimer (compound 4) shown in formula I
[0115]
[0116] The product 3b (921.6 mg, 1.1 mmol) was dissolved in 15 mL of toluene, and commercially available thiophene-2,5-di-tributyltin (332.1 mg, 0.5 mmol) and tetrakis(triphenylphosphine) palladium (34.7 mg , 0.03mmol), under the heating condition of 120°C, the reaction was stirred for 36 hours. The toluene in the reaction system was distilled off under reduced pressure to obtain a red-black solid, which was dissolved in a mixed solvent of 50 mL of dichloromethane and 50 mL of water, the dichloromethane layer was collected by liquid separation, and the dichloromethane was distilled off under reduced pressure to obtain a red A black solid was passed through a H60 silica gel column to obtain product 4 (727.5 mg, 0.455 mmol, yield: 91%).
[0117] The structural characterization data of this product are as follows:
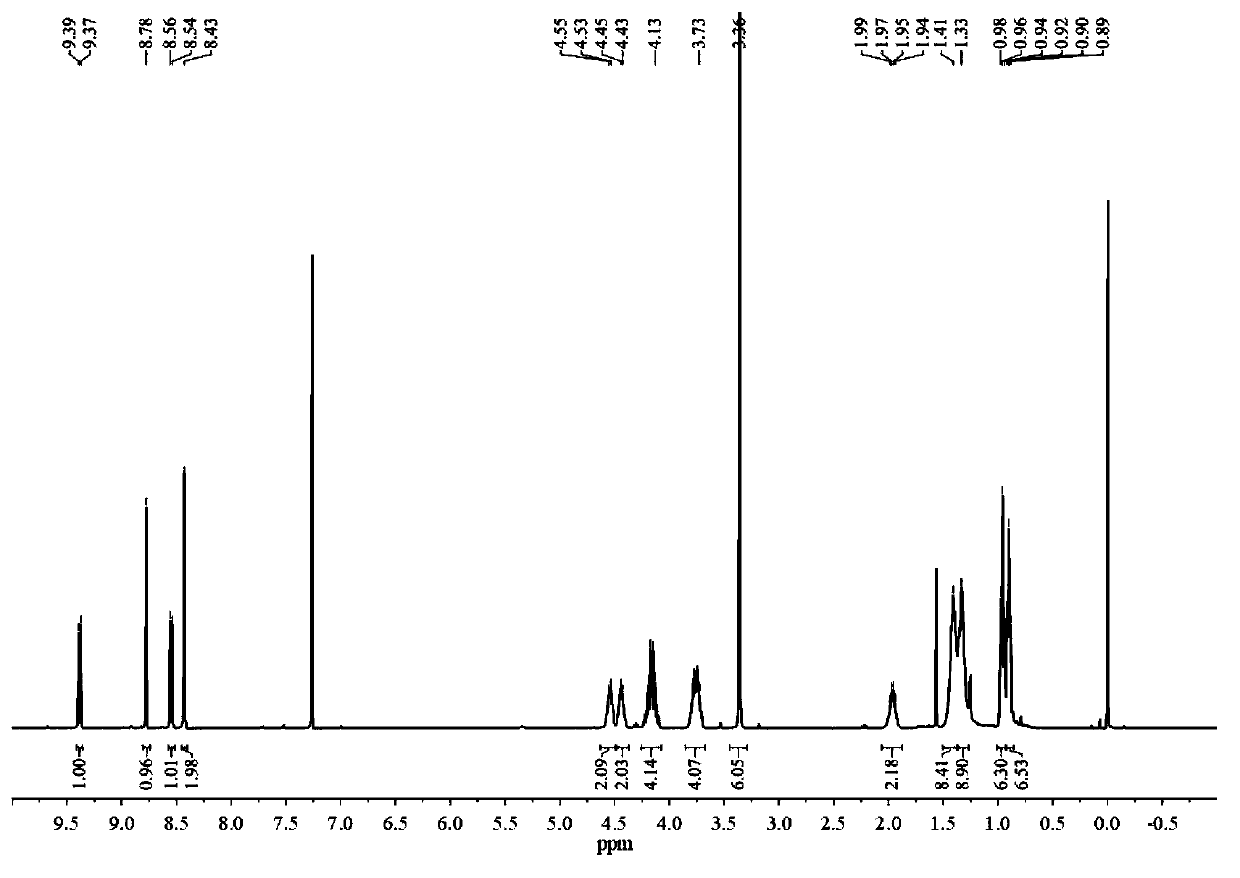
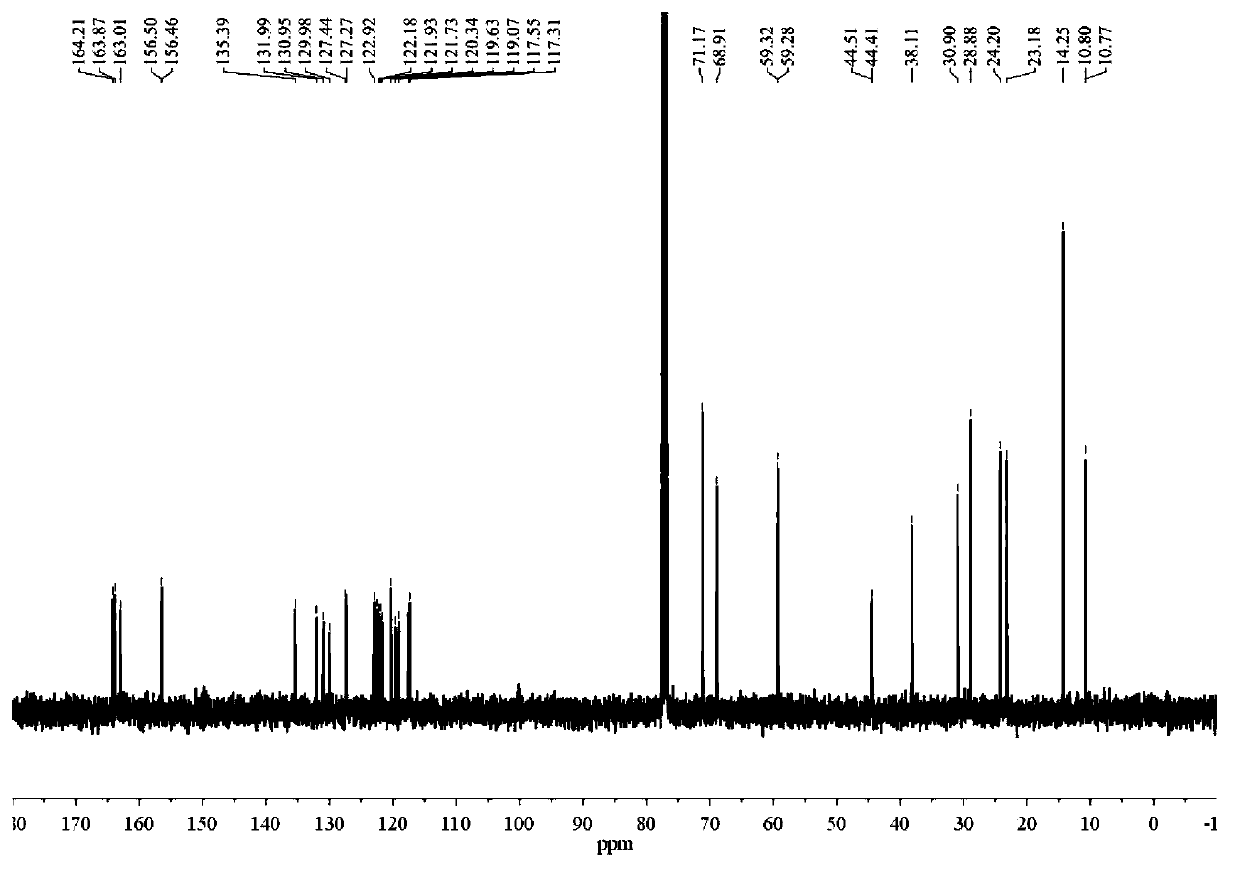
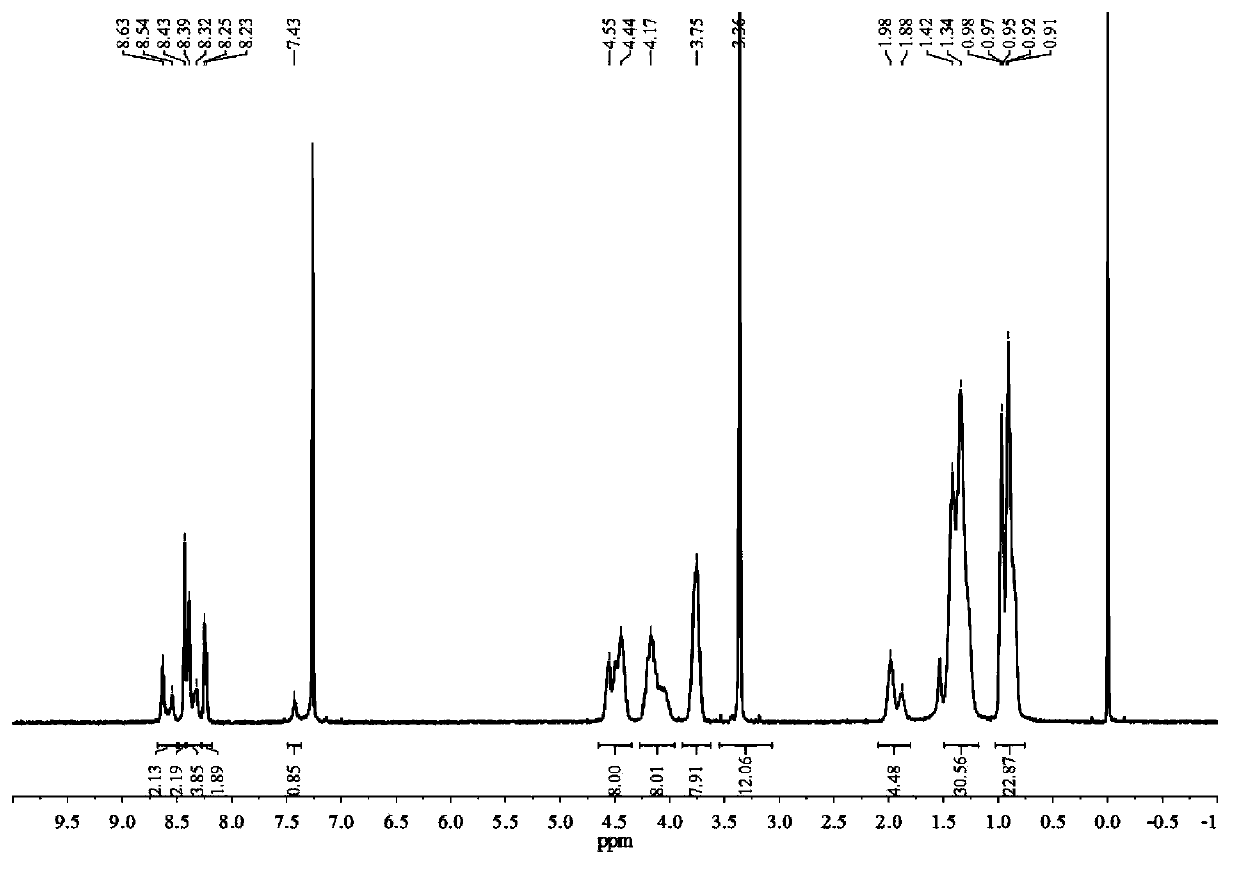
[0118] 1 H-NMR (400MHz, CDCl 3 )δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com