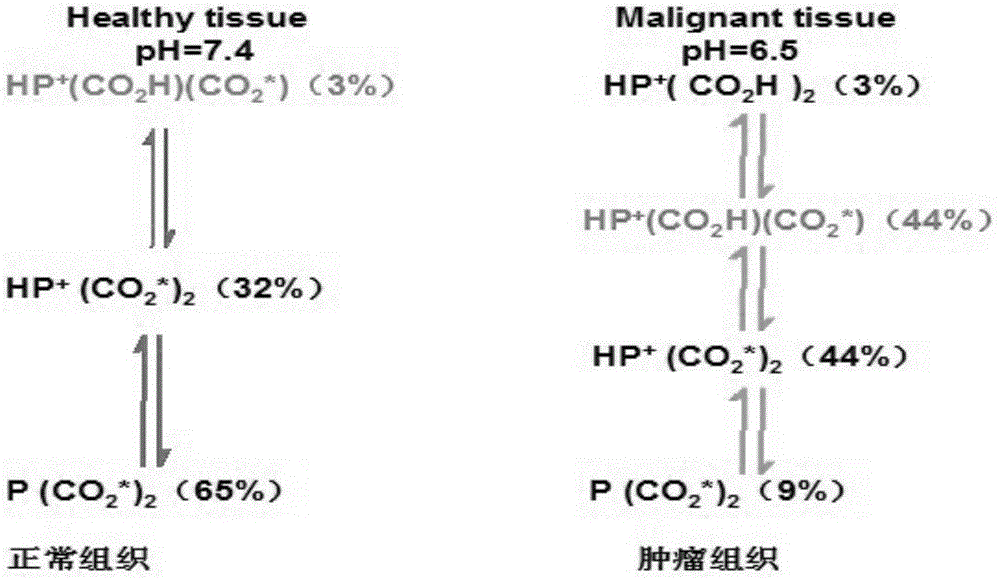
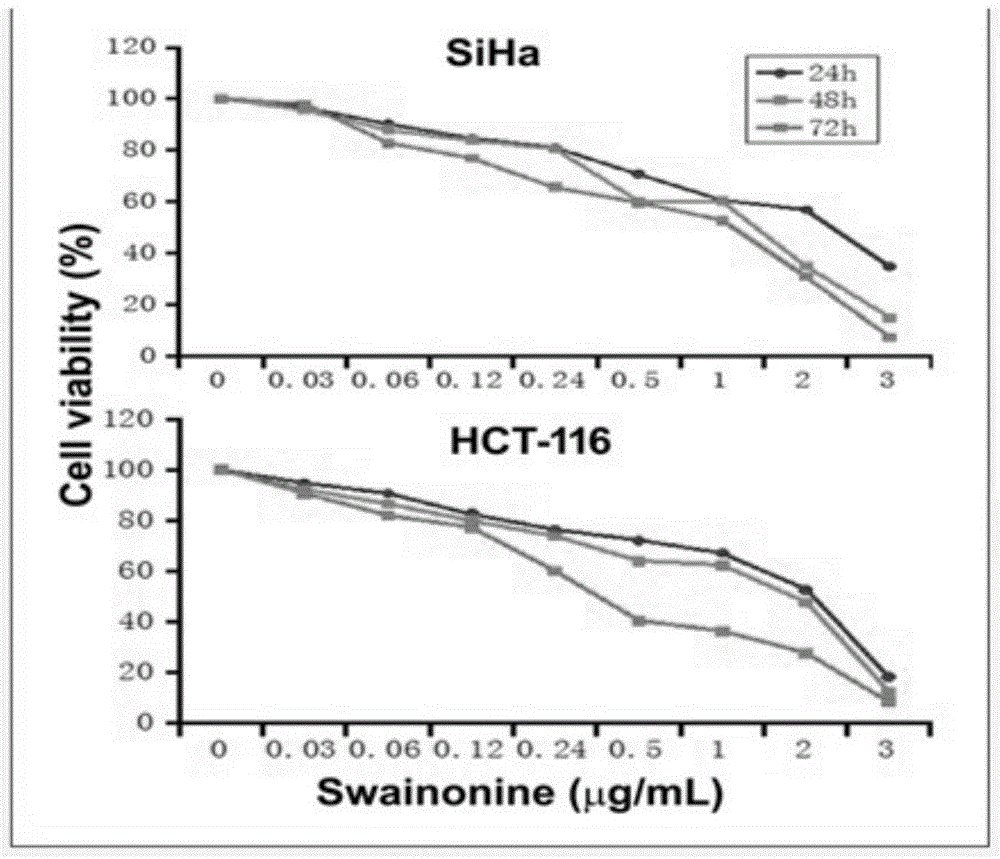
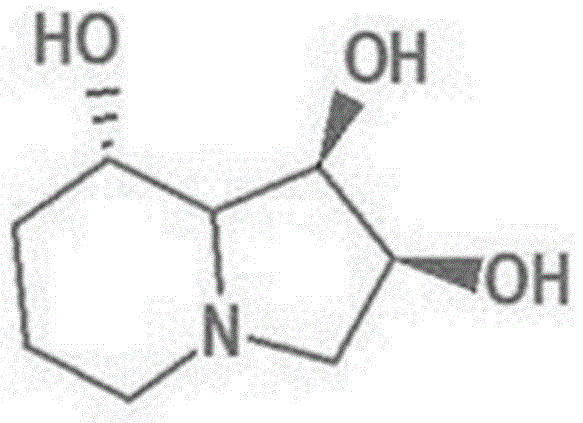
Swainsonine derivative and preparation method of research reagent of swainsonine derivative
A technology of swainsonine and derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of toxicity and unconnected porphyrin rings, so as to eliminate hidden dangers and produce spectacular production benefits , to avoid the effect of hepatotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0055] The present invention will be further described below in conjunction with specific embodiments.
[0056] (1) Drugs (analytical pure) used in this example
[0057] Pure swaintonin (imported from Germany) Shanghai Haring Biotechnology Co., Ltd.
[0058] Protoporphyrin Shanghai Haling Biotechnology Co., Ltd.
[0059] Cordyceps sinensis (cordycepin) Shanghai Shifeng Biotechnology Co., Ltd.
[0060] β-Cyclodextrin Shanghai Haling Biotechnology Co., Ltd.
[0061]
[0062] The guest molecules with high solubility and organic solvents promote the separation of precipitates and inclusions.
[0063] For guest molecules that are insoluble in water, add a small amount of solvent to dissolve them and then add them to a saturated solution.
[0064] If the inclusions are precipitates, filter and wash with water, take a small amount of appropriate organic solvent to wash away the residual drug, and dry to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com