Substituted benzamide liver X receptor (LXR) agonists and application
A technology of benzamide and agonist, applied in the pharmaceutical composition of the compound, the field of agonist of liver X receptor, can solve the problem that the mechanism has not yet been elucidated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
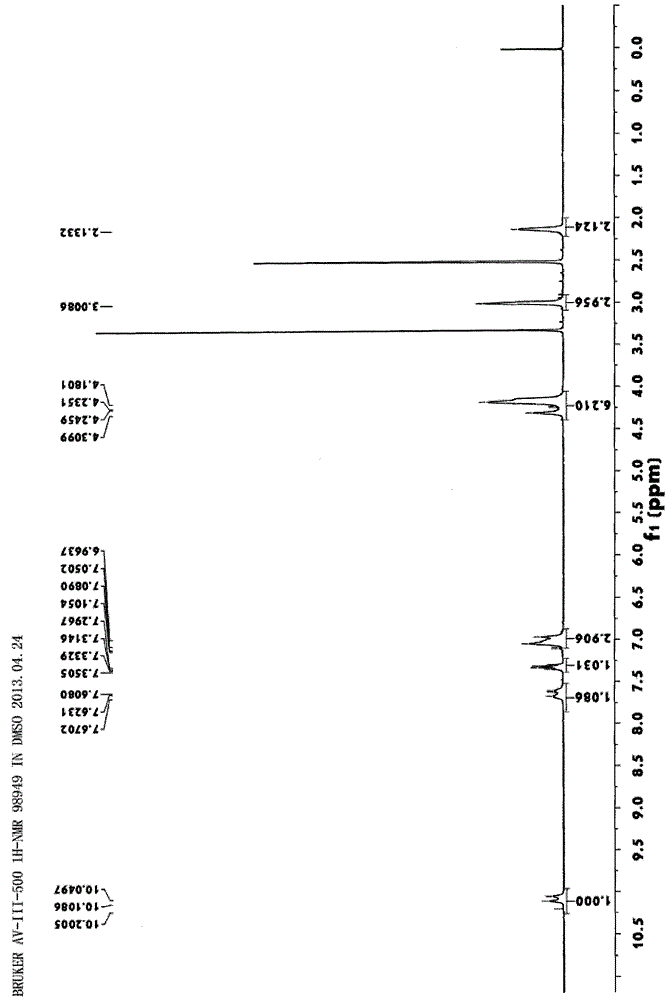
[0090] Example 1. N-methyl-N-(2-oxo-2-(2,3,4-trifluoroanilino)ethyl)-3,4-dihydrobenzo-1,5-diox Synthesis of Heterocyclic-7-Carboxamides
[0091]
[0092] Take 2,3,4-trifluorobenzamide (5.90g, 0.04mol) in a 100mL three-necked flask, add 40mL of tetrahydrofuran to dissolve it, and add 15mL of chloroacetyl chloride (4.52g, 0.04mol) dropwise at 0°C ) tetrahydrofuran solution, add 4.2mL (0.06mol) triethylamine after the dropwise addition, and stir at room temperature for 4h; 2 O (8.73g, 0.04mol) was stirred at room temperature for 12h. After evaporating the solvent, dissolve it with 40mL of dichloromethane, add 10mL of ammonia water, and react at reflux at 90°C for 12h. After the reaction is completed, evaporate to dryness and dissolve to obtain a yellow solid, dissolve it with 30ml of 5% hydrochloric acid at low temperature, wash the water phase with 20ml of ethyl acetate, then adjust the pH to 9 with saturated sodium carbonate in an ice bath, and precipitate a large amount o...
Embodiment 2
[0097] Example 2. Culture of cells
[0098] Both human liver cancer cells HepG2 and human colorectal adenocarcinoma cells Caco-2 are adherent cells. HepG2 was passaged every 48 hours, and Caco-2 was passaged every 96 hours. After the cells are full, discard the old medium, rinse the cells with PBS and discard, then add an appropriate amount of trypsin, digest HepG2 at room temperature for about 2 minutes, and digest Caco-2 at 37°C for 10 minutes, discard the digestion solution, and immediately Add complete MEM medium containing 10% FBS to inhibit the activity of trypsin, gently blow and tap the cells in the culture flask with an elbow pipette to completely detach the cells from the bottom of the bottle and disperse them into a single cell suspension by blowing and blowing. Then inoculate the cell suspension in a new cell flask at a ratio of 1:3, then add an appropriate amount of complete medium, and put it in an incubator to continue culturing. Culture conditions: 37°C, 5% CO...
Embodiment 3
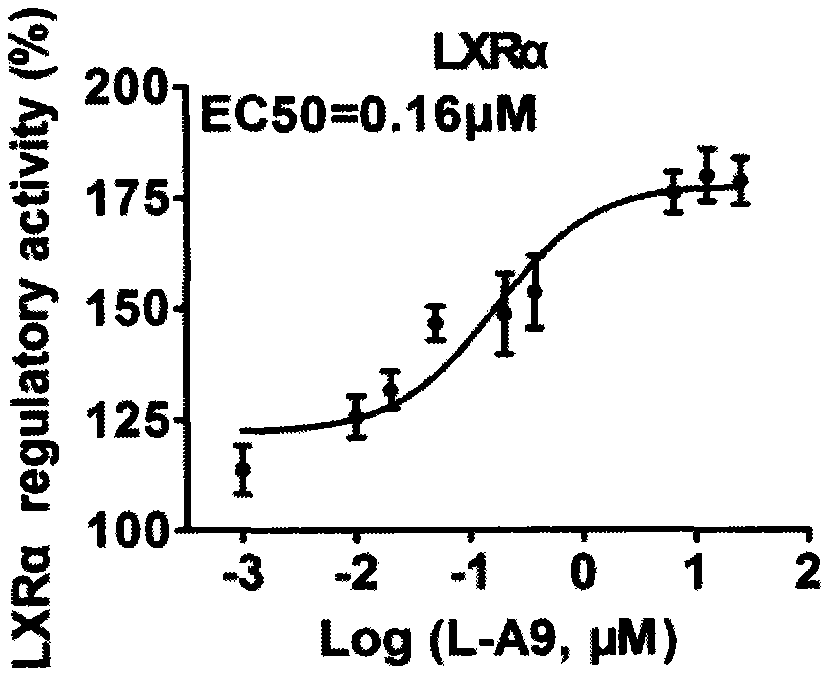
[0101] Example 3. Determination of compound (I) agonistic activity on LXR
[0102] In this activity determination, the agonist screening model of LXR is used to measure the activity of the compound.
[0103] Measuring principle:
[0104] The principle of this assay is that, based on the two main domains in the LXR structure: Ligand Binding Domain (LBD) and DNA Binding Domain (DBD), and the fact that the transcription factor GAL4 in yeast has a similar structure to nuclear receptors, the application According to the principle of yeast two-hybrid, two plasmids are constructed respectively: pBIND-LXR plasmid contains the DBD part of the GAL4 gene and the LBD domain of LXR, which can change conformation when activated by ligand; the other plasmid is pGL4-GAL4, which contains the GAL4 gene Luciferase reporter gene Luc from the promoter region response element. We co-transfected the two plasmids into HepG2 cells to study the activation of LXR by the compounds. In this detection s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com