Application of fingolimod and analogue thereof in preparing medicines for treating cerebral infarction
A technology with a similar structure to fingolimod hydrochloride, applied in the field of fingolimod and its structural analogs, can solve the problems of no cerebral infarction and achieve the effect of expanding clinical indications, significant curative effect, and highlighting the promotion prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Preparation of drugs
[0028] Take 50g of fingolimod and 2450g of starch, mix 950g of starch with water to make a thin slurry, then gradually add the remaining starch and all of fingolimod to the slurry under stirring, stir all the ingredients evenly, make granules, and then Sieve, dry, and pack into capsules.
[0029] 2. Selection of experimental objects
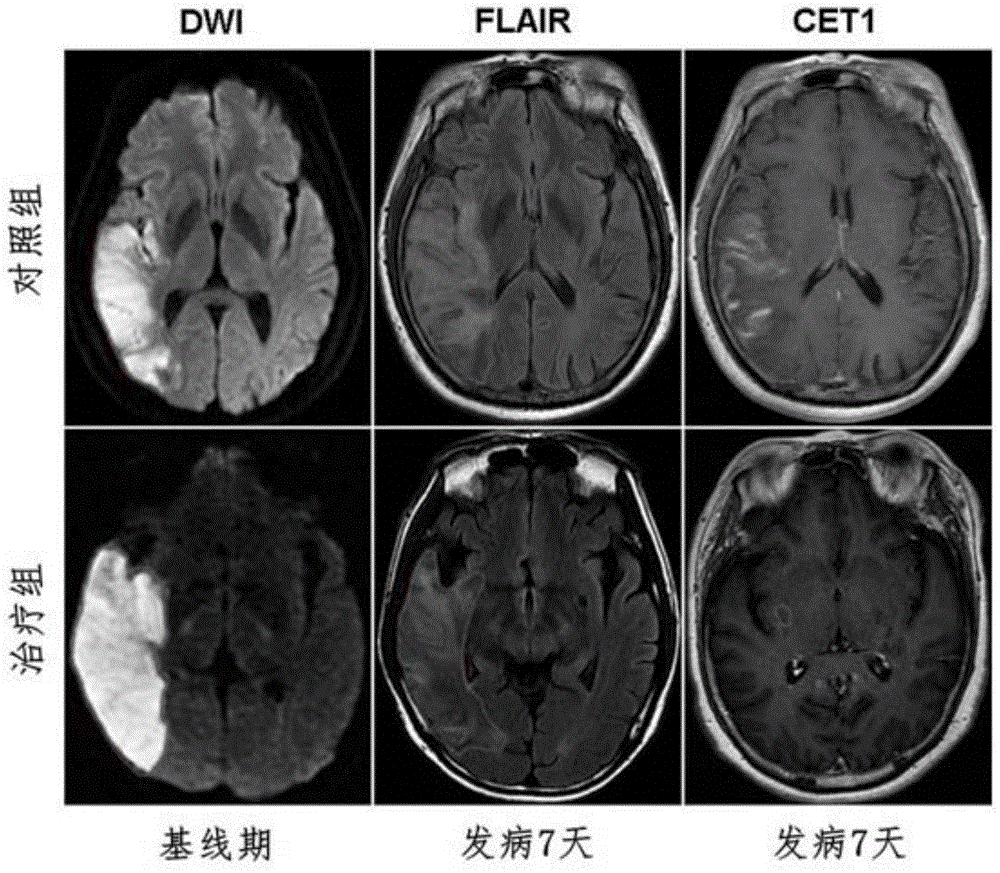
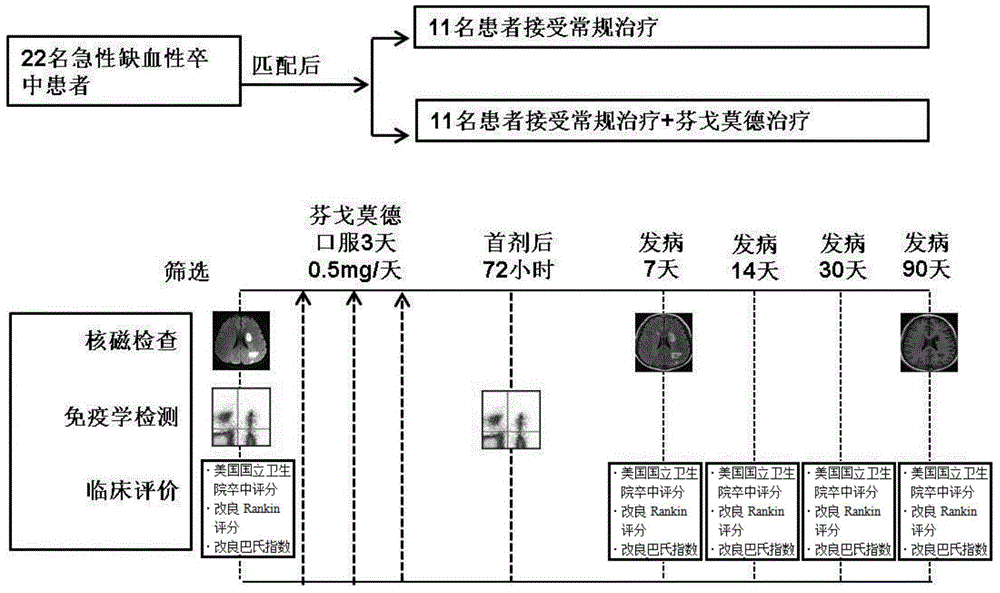
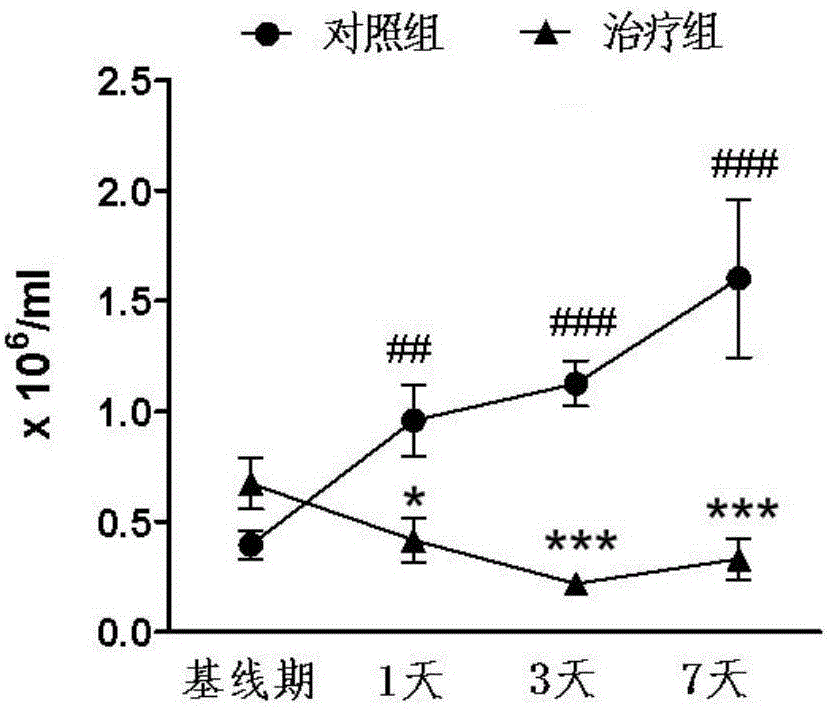
[0030] From March 2013 to December 2013, 11 patients with onset time of more than 4.5 hours but less than 72 hours had the first episode of anterior circulation, the infarct volume was more than 5ml, and the National Institutes of Health stroke score was more than 5 points. Patients with lacunar infarction, without arrhythmia, macular edema, tumors and immunosuppressants were used as experimental subjects, and 11 matched controls with clinical and imaging characteristics.
[0031] 3. Experimental method
[0032] Patients were divided into fingolimod treatment group and control group, the control group received ...
Embodiment 2
[0048] Take 50g of fingolimod and 2450g of starch, mix 950g of starch with water to make a thin slurry, then gradually add the remaining starch and all of fingolimod to the slurry under stirring, stir all the ingredients evenly, make granules, and then Sieve and dry to form granules.
Embodiment 3
[0050]Take 50g of fingolimod, 2300g of starch, and 150g of magnesium stearate, mix 950g of starch and water to make a thin slurry, and then gradually add the remaining starch, all of fingolimod and all of the stearin to the slurry while stirring Magnesium acid, all stirred evenly, dried and compressed into tablets, film-coated to form tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com