Conversion method for dimers
A compound and reaction system technology, applied in the field of conversion and utilization of dimer impurities, can solve the problems of increased production cost, low yield, affecting the quality of final product linagliptin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
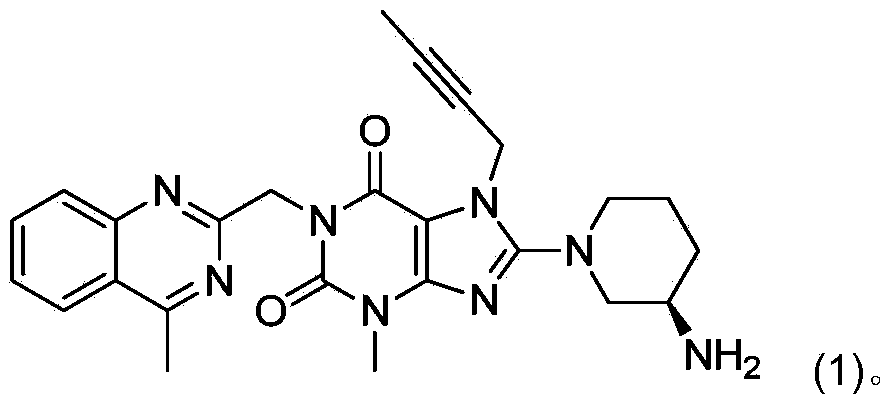
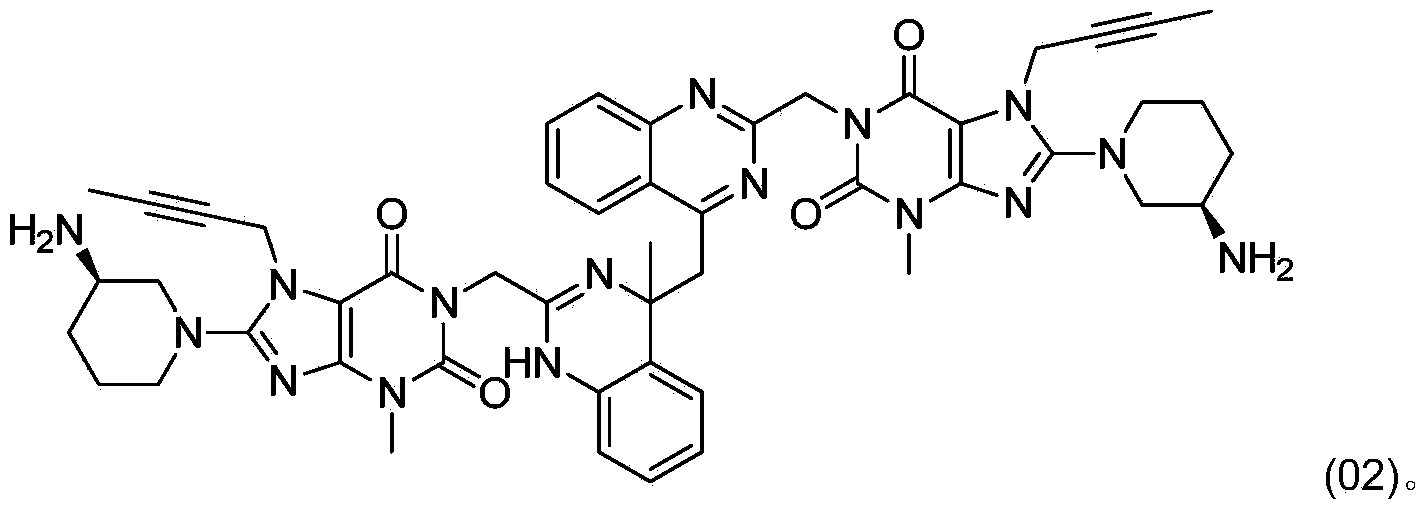
[0007] In the preparation process of linagliptin obtained by deprotecting the acid group, a dimer impurity compound (02) is easily generated, and its content can often exceed 5%. Its structure is shown in the following formula (02):
[0008]
[0009] Through research, the inventor has developed a method for converting the dimer impurity compound (02) into linagliptin, which can not only remove impurities, reduce by-products, but also increase the yield, which is beneficial to production and cost control.
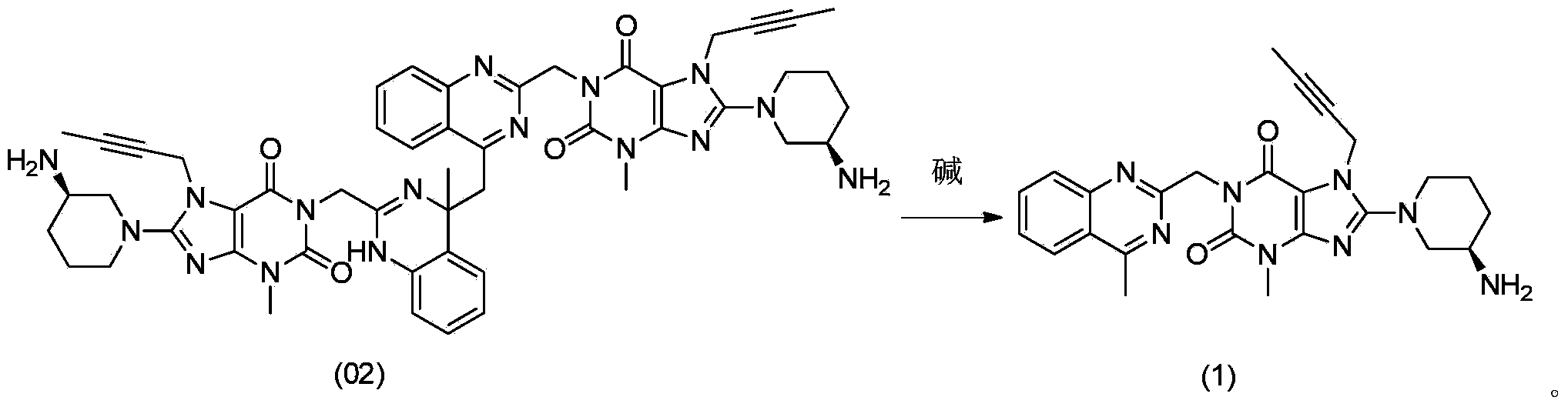
[0010] The method for converting dimer impurity compound (02) into linagliptin, namely compound (1), comprises: under the condition of adding an alkaline reagent, compound (02) is converted into Linagliptin:
[0011]
[0012] The reaction solvent is: N,N-dimethylformamide, dichloromethane, toluene, methanol, ethanol, isopropanol, acetonitrile, tetrahydrofuran, 2-methyltetrahydrofuran, 1,4-dioxane, acetone , butanone, 3-pentanone, ethyl acetate, water, or combinations ...
specific Embodiment approach
[0024] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0025] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0026] In the present invention, g means gram, and mL means milliliter.
Embodiment 1
[0028] In the reaction flask, add 2.8 g of linagliptin crude product containing 13.8% dimer, add 15 mL of dichloromethane, add 1.5 mL of triethylamine to adjust the pH of the system to 8-9, heat to reflux for 24 hours, and detect by HPLC that it contains 1.36% dimer, stop heating, transfer to room temperature and stir for 0.5 hours, then cool to 0°C, crystallize for 1 hour, then filter and dry to obtain 2.5g of solid product linagliptin; HPLC detection product does not contain dimer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com