Pharmaceutical composition containing as active ingredient extract from bark of liriodendron tulipifera
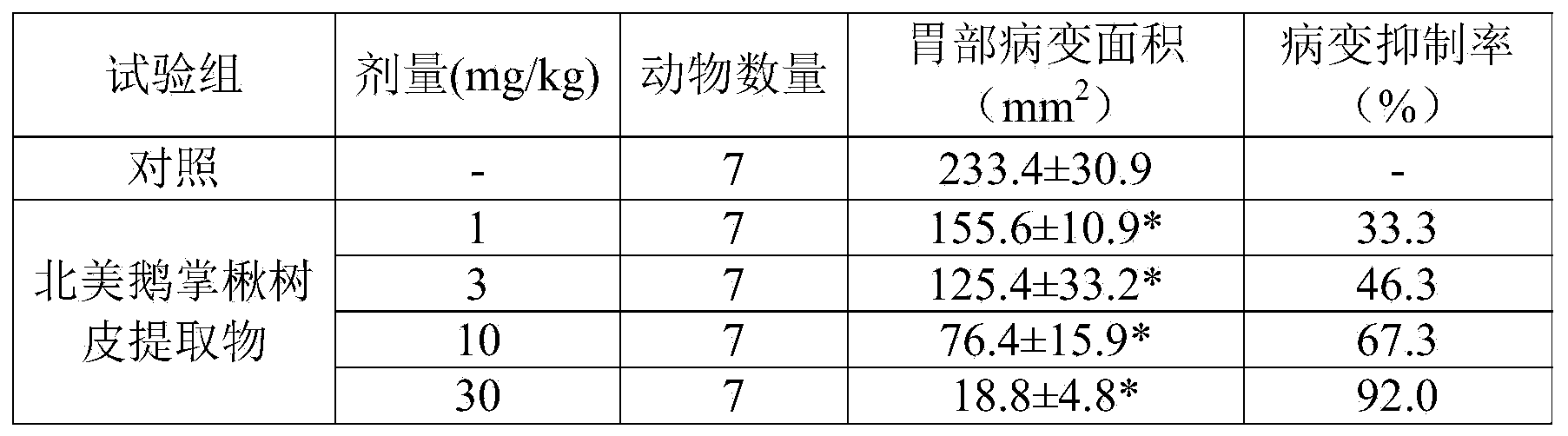
The technology of an active ingredient, epitulipriolide, applied in the field of pharmaceutical compositions containing the bark extract of Liriodendron tulipifera as an active ingredient, can solve the problem that the cause of gastritis and/or gastric ulcer has not been clearly disclosed, and the Development of treatment methods, complicated causes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The method for preparing the bark extract of Liriodendron tulipifera can be explained as follows.
[0045] The preparation method of the present application comprises 2 preparation steps: (1) a step (method 1) of preparing the bark extract of Liriodendron tulipifera using a specific solvent of ethyl acetate or dichloromethane, (2) by improving step (1) Purification step (method 2) to obtain purified material from the amount of the component indicator in the extract obtained.
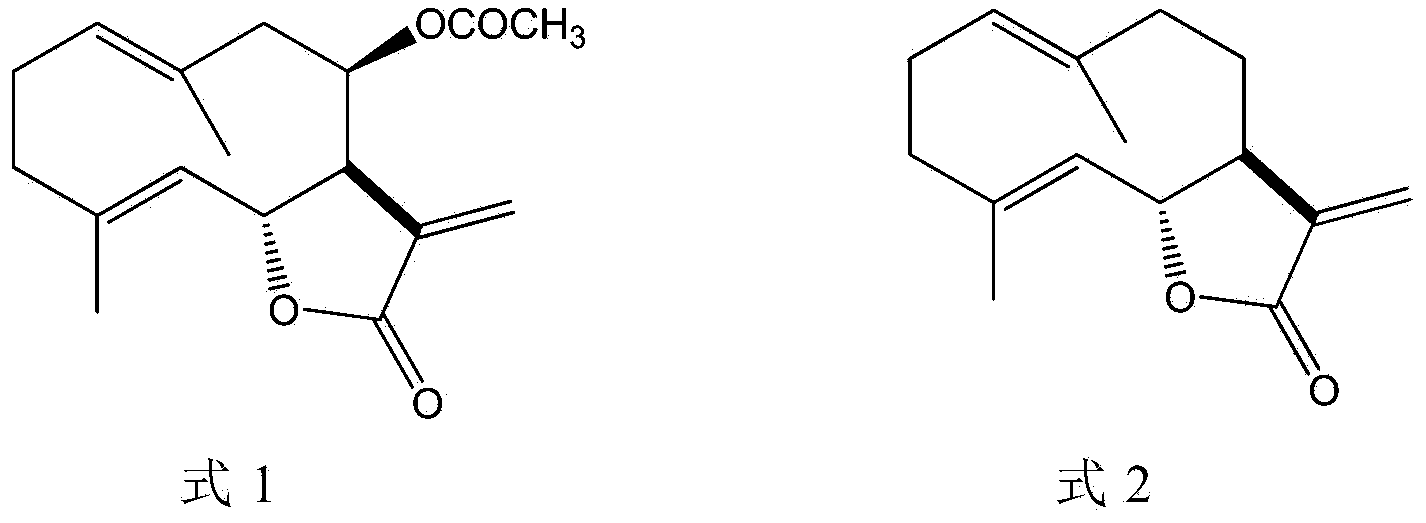
[0046] In method 1, after the bark of Liriodendron tulipifera is chopped and powdered, extraction is performed using an extraction solvent selected from ethyl acetate or dichloromethane. After concentrating the extract, the obtained substance contains epituliprolactone and corylidene as active ingredients.
[0047] In Method 2, the extract obtained in Method 1 was dissolved using an aqueous alcohol solution. After adding hexane, lipid components and water-insoluble substances dissolved in hexane...
preparation example 1
[0063] Preparation Example 1: Preparation of Isolated and Purified Liriodendron bark extract
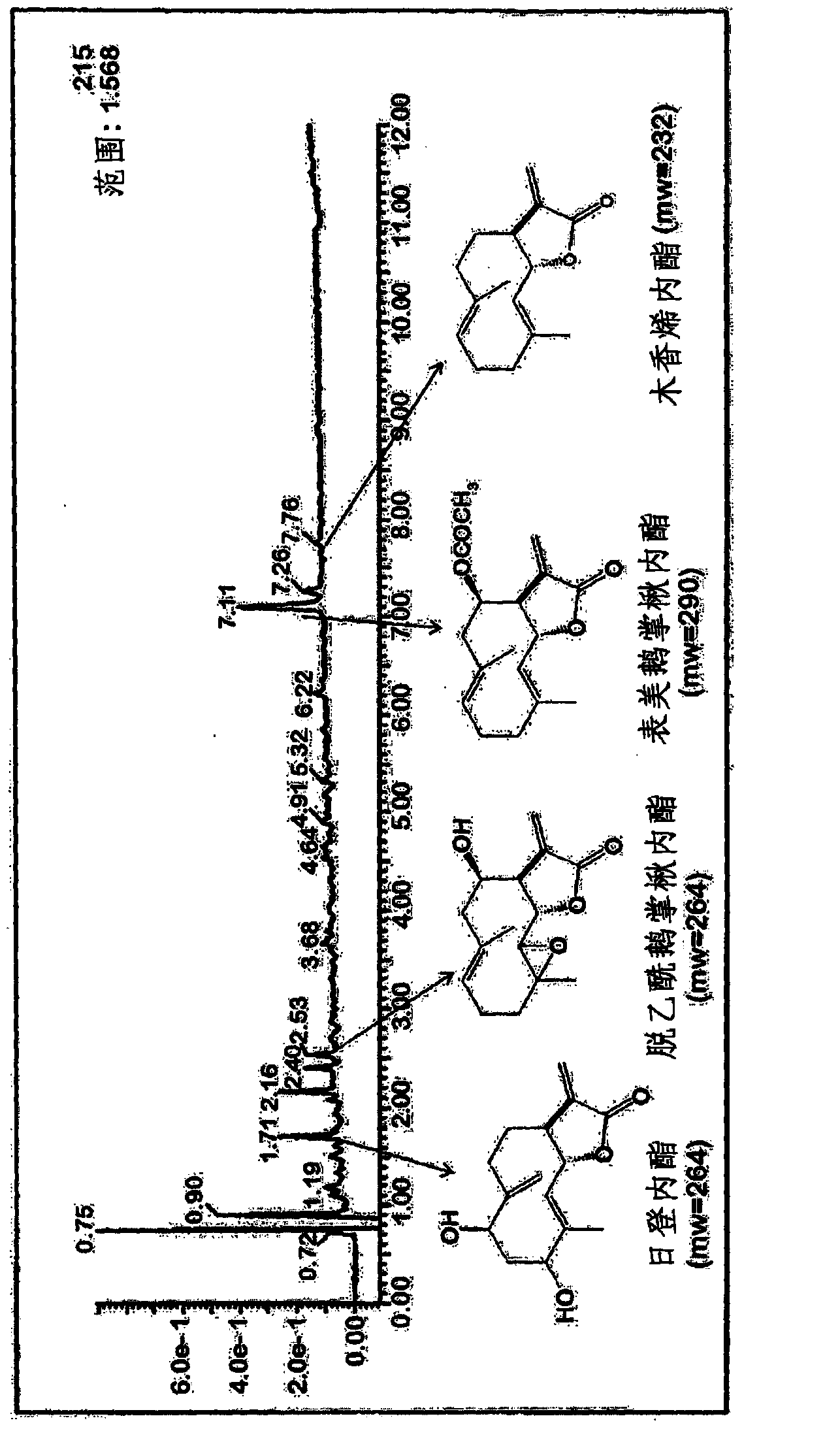
[0064] 200 g of chopped and dried bark of Liriodendron tulipifera was extracted with 800 ml of ethyl acetate for 24 hours at 50°C. The resulting extraction mixture was concentrated and dried under reduced pressure. 6.83 g of extract was finally obtained. The amount of epituliprolactone was 3.96 wt%, and the amount of lignolide was 0.18 wt%.
[0065] Dissolve 1 g of the extract obtained in the above steps with 50 ml of 70% ethanol. After adding 50 ml of hexane, the mixture was stirred and partitioned. The resulting 70% ethanol fraction was separated and dried. Finally, 0.483 g of purified extract was obtained. The amount of epituliprolactone was 9.72 wt%, and the amount of lignolide was 0.28 wt%.
[0066] figure 1 LC-MS chromatographic data of the extract isolated from the bark of Liriodendron tulipifera described in Preparation 1 are shown. It was confirmed that the extract o...
preparation example 2
[0067] Preparation Example 2: Preparation of Isolated and Purified Liriodendron bark extract
[0068]200 g of chopped and dried bark of Liriodendron tulipifera was extracted with 800 ml of ethyl acetate for 48 hours at 50°C. The resulting extraction mixture was concentrated and dried under reduced pressure. 7.67 g of extract was finally obtained. The amount of epituliprolactone was 3.94 wt%, and the amount of lignolide was 0.14 wt%.
[0069] Dissolve 1 g of the extract obtained in the above steps with 50 ml of 70% ethanol. After adding 50 ml of hexane, the mixture was stirred and partitioned. The resulting 70% ethanol fraction was separated and dried. Finally, 0.571 g of purified extract was obtained. The amount of epituliprolactone was 10.94 wt%, and the amount of lignolide was 0.30 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com