Synthetic method of diphosphonate medicine
A bisphosphonate and synthesis method technology, which is applied in the synthesis process and production application field of bisphosphonate drugs, can solve the problems of severe heat release, less supply, and difficulty in the treatment of three wastes, so as to improve economic benefits and reduce The effect of pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
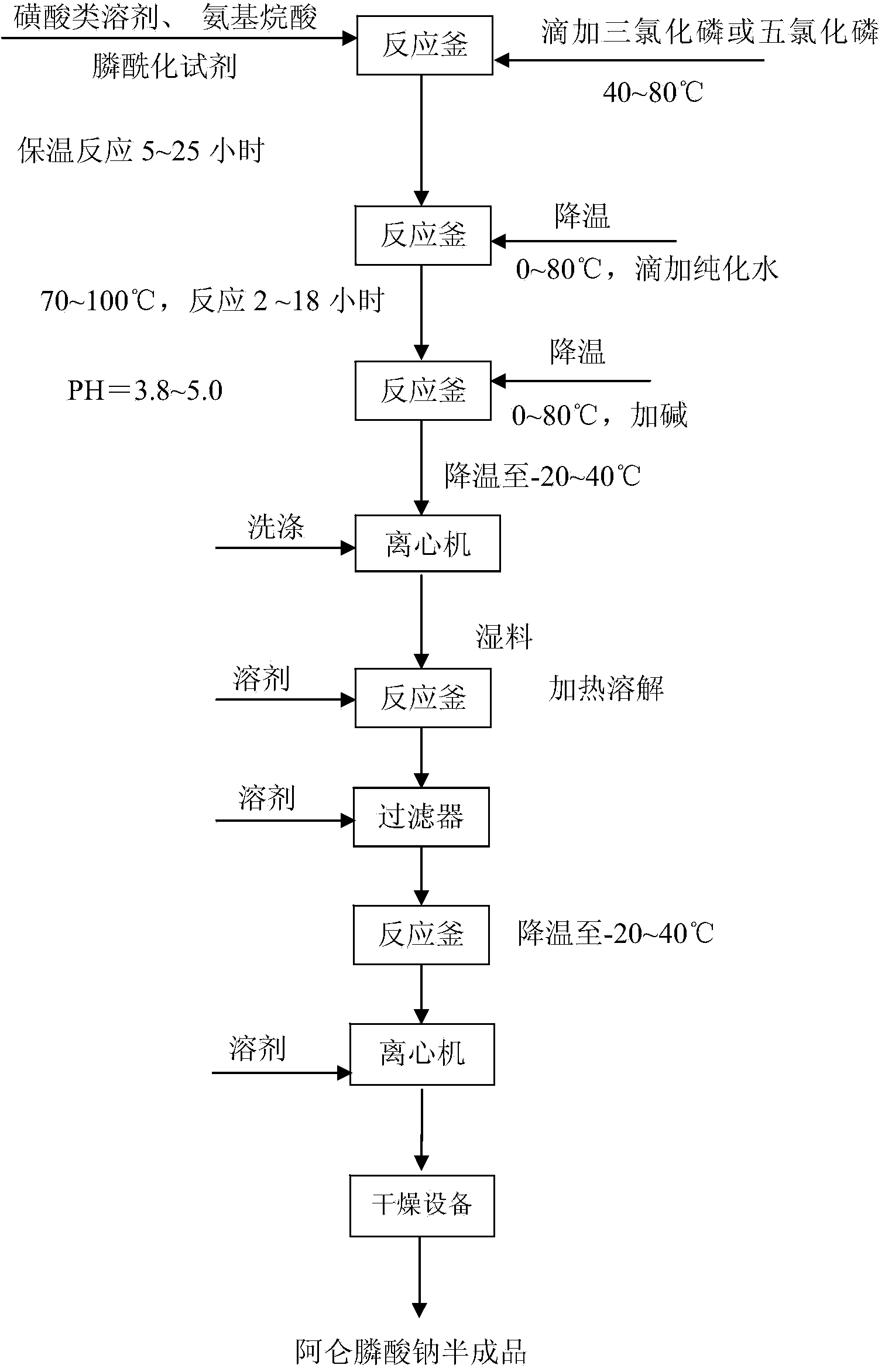
[0033] 1) Preparation of crude bisphosphonates
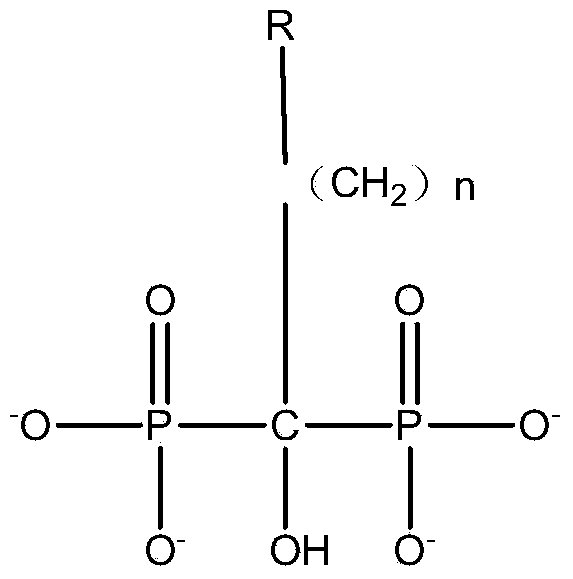
[0034] 1.1] Add methanesulfonic acid, aminoalkanoic acid, phosphonylation reagent (phosphonite, phosphonyl chloride, etc.) -Alanine and other aminoalkanoic acids with substituent groups on the amino group; phosphonylating reagents include but not limited to phosphonyl chloride, sulfurous acid and their esters; methanesulfonic acid can also be used by other sulfonic acid liquid solvents Replaced; the material dissolution temperature range is 30-130°C; the molar ratio of the material is: aminoalkanoic acid: methylsulfonic acid: phosphonylation reagent: phosphorus trichloride / phosphorus pentachloride = 1.0: 3.0 ~ 10.0: 0.6 ~2.0: 1.0~5.0.
[0035] 1.2] Add phosphorus trichloride or phosphorus pentachloride dropwise, the temperature is in the range of 40-80°C, the reaction is mild, and the reaction is kept for 5-25 hours.
[0036] 1.3] After the reaction is completed, add water dropwise. The temperature range for adding water is 0-...
Embodiment 1
[0044] Synthesis of Alendronate Sodium
[0045] 1] Synthesis of crude product of alendronate sodium
[0046] 1.1] Put 750.0g of methanesulfonic acid, 200.0g of 4-aminobutyric acid and 240.0g of phosphorous acid into a 10L reaction bottle; heat up to 40-80°C, add 850.0g of phosphorus trichloride dropwise. 80 ℃ heat preservation reaction for 10 to 20 hours;
[0047] 1.2] Cool down to 5-35°C, add 3000.0g of water, then raise the temperature to 70-100°C, keep warm for 4-10 hours; slowly add about 3000.0g of 50% liquid caustic soda dropwise, adjust the pH to 3.8-4.5, and cool down to 3 ~20°C; filter with suction, wash the filter cake twice with 500.0 g of water, and drain to obtain 813.0 g of crude alendronate sodium.
[0048] 2] Refining of alendronate sodium
[0049] 2.1] Put the crude alendronate sodium obtained in the above step 1.2] into a 3L reaction bottle, 2400.0g of water, heat up to 40-80°C, add 80.0g of activated carbon, continue to heat up to 60-120°C, and keep warm ...
Embodiment 2
[0053] Synthesis of Pamidronate Disodium
[0054] 1) Synthesis of Pamidronate Disodium Crude Product
[0055] 1.1] Put 648.0g of methanesulfonic acid, 200.0g of 3-aminobutyric acid and 207.0g of phosphorous acid into a 10L reaction bottle; raise the temperature to 40-80°C, add 734.0g of phosphorus trichloride dropwise, after the dropwise addition, 40 ~80℃ heat preservation reaction for 10~20 hours;
[0056] 1.2] Cool down to 5-35°C, add 2600.0g of water, then raise the temperature to 70-100°C, keep warm for 4-10 hours; slowly add about 2600.0g of 50% liquid caustic soda dropwise, adjust the pH to 4.4-4.9, and cool down to 3 ~20°C; filter with suction, wash the filter cake twice with 432.0 g of water, and drain to obtain 835.0 g of crude pamidronate disodium.
[0057] 2] Refining of Pamidronate Disodium
[0058] 2.1] Put the crude pamidronate disodium obtained in the above step 1.2] into a 3L reaction bottle, 2500.0g of water, heat up to 40-80°C, add 84.0g of activated carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com